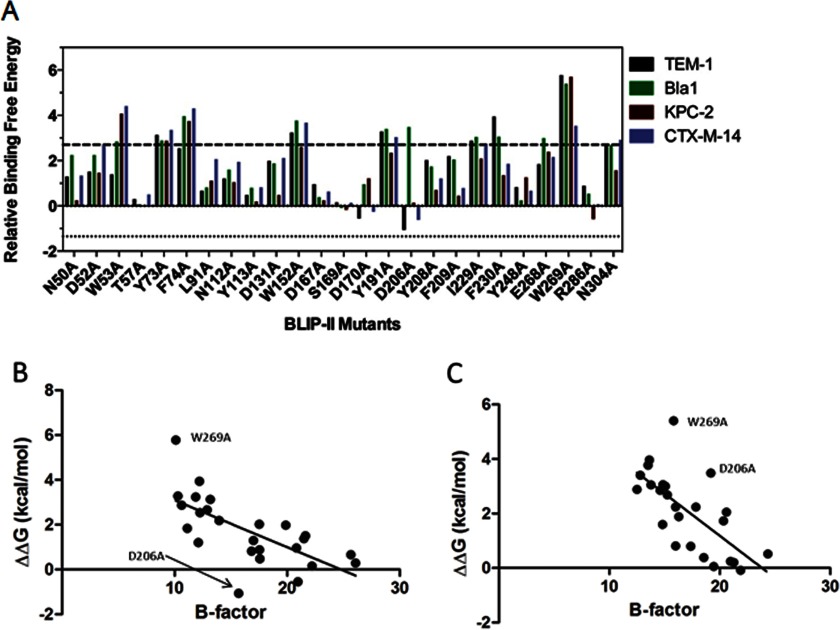
FIGURE 5.
Change in the energetic contributions of the BLIP-II interface residues upon alanine substitution. A, comparison of the ΔΔG values of the BLIP-II alanine variants for binding β-lactamases: TEM-1 (black), Bla1 (green), KPC-2 (red), and CTX-M-14 (purple). Relative binding free energy is defined as the difference in binding energy between wild-type BLIP-II and the alanine mutant (ΔΔG = −RT ln(Kd,wt/Kd,mut)). The upper significance control line is set at 2.7 kcal/mol, which indicates a 100-fold increase in Kd. The lower significance control line is set at 1.35 kcal/mol, which indicates a 10-fold decrease in Kd. B, plot of the temperature factor of BLIP-II interface residues versus the change in binding energy (ΔΔG (kcal/mol)) for the alanine substitution of the positions. The temperature factor for each residue was calculating by averaging the temperature factors for all of the atoms that make up the residue. The ΔΔG values are calculated from the Kd values in Table 2. B, the x axis indicates the temperature factor based on the BLIP-II-TEM-1 β-lactamase x-ray structure (Protein Data Bank 1JTD), and the y axis indicates the change in energy for binding for each mutant to TEM-1 β-lactamase. C, the x axis indicates the temperature factor based on the BLIP-II-Bla1 β-lactamase x-ray structure (Protein Data Bank 3QHY), and the y axis indicates the change in energy for binding for each mutant to Bla1 β-lactamase (27).