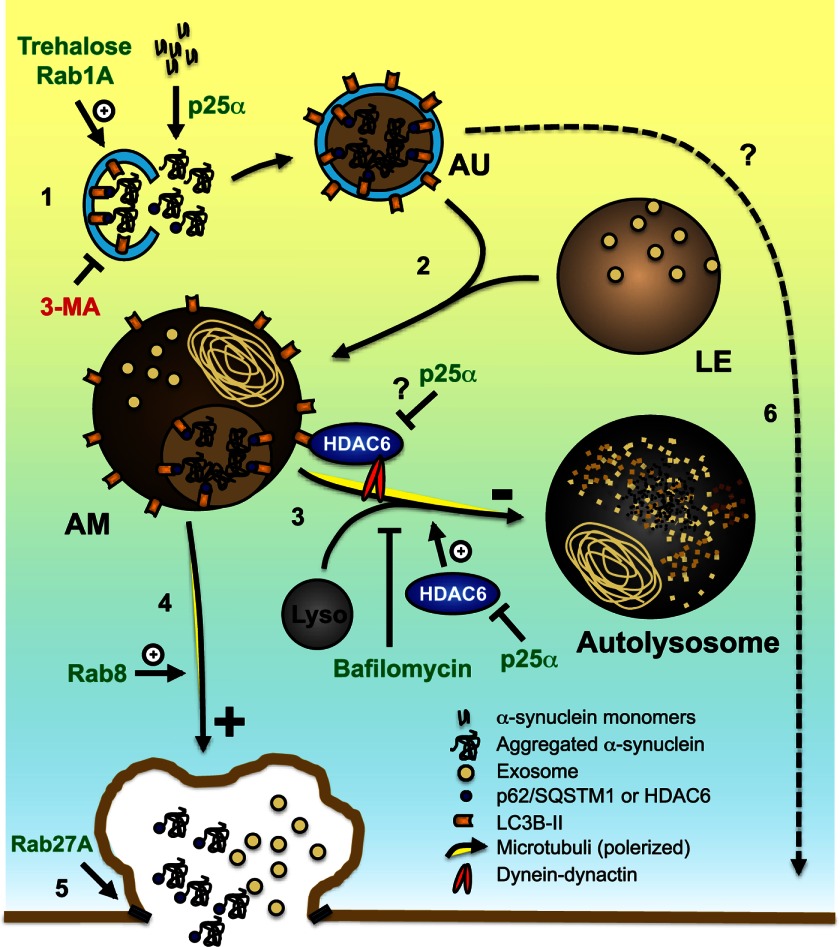
FIGURE 12.
Proposed mechanism for p25α effects and exophagy of α-synuclein. The diagram illustrates how p25α may alter trafficking pathways (marked by numbers) and autophagosome dynamics. Expression of p25α causes aggregation and autophagosomal uptake of α-synuclein (α-SNC), involving QC autophagosome adaptors p62/SQSTM1 and possibly HDAC6 (1). The autophagosome (AU) then fuses (2) with a late endosome (LE) to generate an amphisome (AM), which can travel retrogradely (3) to fuse with a lysosome (Lyso) thereby forming an autolysosome, which degrades α-SNC. Retrograde transport involves HDAC6-mediated interactions between LC3B and the minus-end-directed dynein-dynactin motor complex, which may be inhibited by p25α. Fusion of amphisomes with lysosomes is promoted by HDAC6 deacetylase activity, which is inhibited by p25α. Under conditions where pathway 3 is blocked (compromised HDAC6 activity, lysosomal dysfunction, and/or altered ratio of minus- to plus-end-directed trafficking of amphisomes), anterograde transport of amphisomes toward the cell surface takes place (4), where a fraction of competent amphisomes can undergo exocytosis regulated by Rab27A (5), to release α-SNC in monomer and aggregated/modified forms to the extracellular environment. The extent to which autophagosomes may directly contribute to exocytosis is unclear (6).