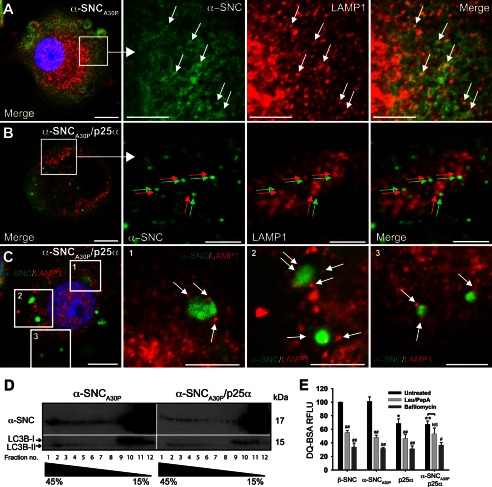
FIGURE 5.
Expression of p25α prevents α-synuclein in reaching lysosomes. A–C, indirect immunofluorescence of leupeptin/pepstatin A-treated PC12 cells expressing α-synucleinA30P (α-SNCA30P) alone (A) or together with p25α (B and C), showing the distribution of α-SNCA30P (Abcam LB509) and the lysosomal marker LAMP1. Arrows in A indicates co-localization between the antigens, and arrows in B (red and green) and C (white) indicate that, although closely apposed, α-SNC immunoreactivity is distinct from that of LAMP1. Bars, A–C, 10 μm in the left panels and 5 μm in close ups. D, homogenates from PC12 cells expressing α-SNCA30P or α-SNCA30P/p25α were fractionated on a 15–45% sucrose gradient and aliquots of collected fractions analyzed by Western blotting of α-SNC (BD Transduction Laboratories) and LC3B. The blots are representative of two independent experiments. Note that p25α decreases the amount of α-SNC present in heavy fractions 1–3, which also contains exclusively autophagosome-associated LC3B-II. E, flow cytometric analysis of PC12 cells incubated with DQ-BSA for 6.5 h with or without either bafilomycin A1 (100 nm) or leupeptin (50 μg/ml)/pepstatin A (67 μg/ml) treatment. DQ-BSA fluorescence intensity was normalized to β-synuclein (β-SNC)-expressing PC12 cells, and the bar graph shows mean ± S.E. of relative fluorescence units (RFLU) (n = 3). * denotes a statistic significant decrease in relative fluorescence units for untreated cell lines when compared with untreated β-SNC-expressing cells; # denotes statistic significant decrease within the same cell line after chemical treatment; NS, nonsignificant.