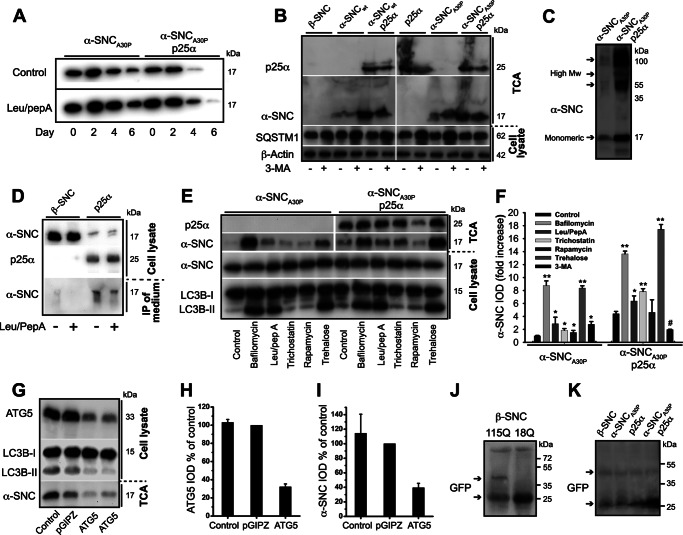
FIGURE 7.
TPPP/p25α induces α-synuclein secretion, which can be modified by regulators of the autophagosomal degradation pathway. A, PC12 cells expressing α-synucleinA30P alone (α-SNCA30P) or with p25α (α-SNCA30P/p25α) were predifferentiated with NGF for 2 days and transgene expression induced for additionally 2 days. Doxycycline was then withdrawn, and the cells chased for 6 days with or without leupeptin (50 μg/ml) and pepstatin A (67 μg/ml). Representative Western blots using anti-α-SNC (BD Transduction Laboratories) shows clearance of α-SNC from cell lysates acquired from day 0, 2, 4, and 6 after doxycycline withdrawal. B, representative Western blots using anti-α-SNC (BD Transduction Laboratories), p62/SQSTM1 (SQSTM1), and β-actin of cell lysates and TCA-precipitated conditioned media obtained from PC12 cell lines expressing β-SNC, α-SNC wild type (α-SNCwt), or α-SNCA30P with or without p25α in the presence or absence of 3-MA (10 mm) (n = 3). C, representative Western blot of TCA-precipitated medium obtained from PC12 cells expressing α-SNCA30P alone or together with p25α using mouse anti-α-SNC (BD Transduction Laboratories). Notice the presence of both monomeric (17 kDa) and high molecular weight (High Mw) forms of secreted α-SNC. D, PC12 cells expressing β-SNC (as control) or p25α were treated or not with leupeptin/pepstatin A (50 and 67 μg/ml) for the last 24 h of culture before analysis of endogenous α-SNC in cell lysates and immunoprecipitates from conditioned medium (using BD Transduction Laboratories and LB509 mAbs) by Western blotting (using anti-α-SNC rabbit mAb EP1646Y) as indicated. Data are representative of three independent experiments. E, PC12 cells expressing α-SNCA30P or α-SNCA30P/p25α were treated with bafilomycin A1 (15 nm), leupeptin (50 μg/ml)/pepstatin A (67 μg/ml), trichostatin A (20 μm), rapamycin (0.5 μm), trehalose (100 mm), or left untreated (control) for 48 h concurrently with doxycycline induction before Western blot analysis of TCA-precipitated conditioned media and cell lysates using antibodies as indicated. F, bar graph shows fold increase in integrated optical density (IOD) of TCA Western blot bands of secreted α-SNC (BD Transduction Laboratories) obtained from B and E relative to untreated PC12-α-SNCA30P cells. Mean ± S.E. of three independent experiments are shown. G, TCA-precipitated conditioned medium or cell lysates from PC12-α-SNCA30P/p25α cells with or without stable co-expression of either control (pGIPZ) or ATG5 shRNA were analyzed by Western blotting using anti-α-SNC (BD Transduction Laboratories), ATG5, or LC3B antibodies as indicated. H, bar graph shows IOD of ATG5 western bands normalized to PC12 cells expressing control shRNA (pGIPZ) and represents mean ± S.E. of three independent experiments. I, bar graph shows IOD of α-SNC western bands (TCA samples) normalized to PC12 cells expressing control shRNA (pGIPZ) and represents mean ± S.E. of three independent experiments. J and K, PC12 cell populations as indicated were transduced with either Htt-18Q-GFP or Htt-115Q-GFP for 2 days before transgene induction for a further 2 days. Conditioned medium was then TCA-precipitated and analyzed by Western blotting with polyclonal rabbit anti-GFP antibodies. J, a ∼50-kDa band (upper arrow) immunoreactive with anti-GFP antibodies is seen exclusively in the conditioned medium from HTT-115Q-GFP-expressing cells but not from control HTT-18Q-GFP cells. K, similar secretion of HTT-115Q-GFP was observed in PC12 cells expressing β-SNC, p25α, or α-SNCA30P with or without p25α expression. The increased reactivity to monomer GFP to the right on the blot is caused by overflow from an adjacent (data not shown) cell lysate lane. Upper and lower arrows indicate HTT-115Q-GFP fusion protein and monomeric GFP, respectively.