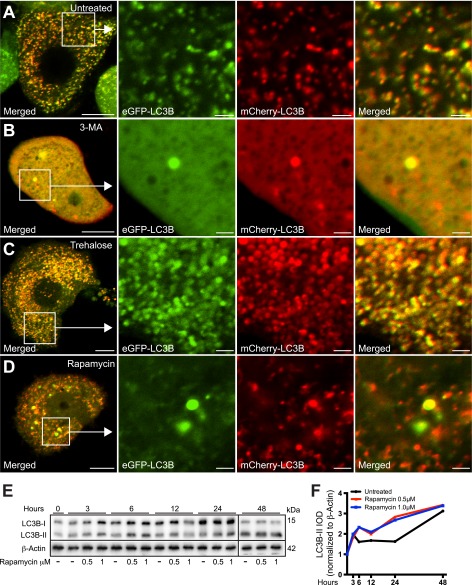
FIGURE 8.
mTOR-dependent and -independent autophagy enhancers rapamycin and trehalose, respectively, differentially affect the distribution and fluorescence properties of mCherry-eGFP-LC3B. A–D, PC12-α-synucleinA30P/p25α (α-SNCA30P/p25α) cells expressing mCherry-eGFP-LC3B were treated with 3-MA (10 mm), trehalose (100 mm), or rapamycin (0.5 μm) as indicated, for the last 48 h of culture, before live imaging with a Zeiss LSM510 confocal microscope. Note that 3-MA causes the diffusive cytoplasmic distribution of the mCherry-eGFP-LC3B construct, whereas trehalose induces the massive accumulation of large autophagosomal vacuoles that emit both mCherry and GFP fluorescence. In contrast, autophagy induced by rapamycin increased the proportion of autophagosomal vacuoles with predominant emission of only mCherry-fluorescence indicating correct acidification. Bars, 10 μm, enlarged images 2 μm. The images shown are representative of three independent experiments. E, PC12-α-SNC/p25α cells were treated with 0.5 or 1 μm rapamycin for different time intervals as indicated, and cell lysates were then analyzed by Western blotting for conversion of LC3B and levels of β-actin (loading control). Samples from the 48-h time point were run on separate gel due to lack of wells. The Western blot is representative of two independent experiments. F, integrated optical density (IOD) of LC3B-II bands from the experiment shown in E were normalized to levels of β-actin and plotted over time.