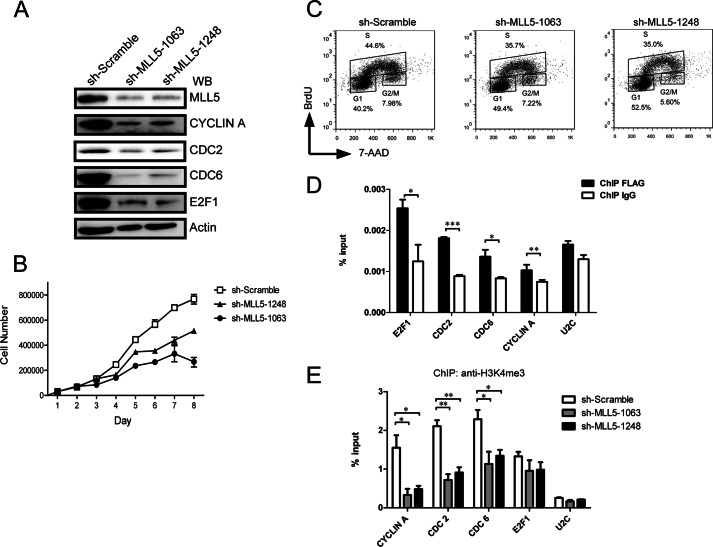
FIGURE 5.
Knockdown of MLL5 caused cell proliferation defects, and the levels of both H3K4 trimethylation at E2F1-responsive promoters and the E2F1 target gene expression are down-regulated in MLL5 knockdown cells. A, knockdown of MLL5 protein in HeLa cells leads to down-regulation of E2F1 target genes. Two different lentivirus-based shRNAs target to an mRNA sequence starting 1063 and 1248 bp downstream of the translation start site were used to knockdown MLL5 expression in HeLa cells. Whole cell lysates were harvested at day 6 post-infection. The expression of endogenous E2F1, CYCLIN A, CDC2, and CDC6 were detected by polyclonal antibodies. Actin was used as a loading control. B, defective cell proliferation in MLL5 knockdown cells. 3 × 104 cells were seeded in 48-well plates at day 1, at each indicated time point, triplicates of cells from each group were harvested and counted in a hemocytometer. C, BrdU incorporation assay in MLL5 knockdown cells. BrdU was added to the culture medium for 1 h, and cells were collected, fixed, and then stained with anti-BrdU antibody conjugated with allophycocyanine, and 7-aminoactinomycin D (7-AAD) was used to stain genomic DNA. D, MLL5 localizes to E2F1-responsive promoters. HEK293T cells were transiently transfected with plasmids encoding 3× FLAG-tagged MLL5, and the cells were harvested 48 h later. Quantitation of ChIP analysis of transfected HEK293T cells with an anti-FLAG antibody by triplicate real-time PCR of the indicated promoters is shown. E, the H3K4 trimethylation levels at E2F1 target promoters were decreased significantly in MLL5 knockdown cells. Quantitation of H3K4 trimethylation ChIP analysis of HeLa cells infected with lentivirus-based scramble control or shRNA by triplicate real-time PCR of the indicated promoters is shown. Two-tailed unpaired Student's t tests were performed, *, p < 0.05; **, p < 0.01; ***, p < 0.001.