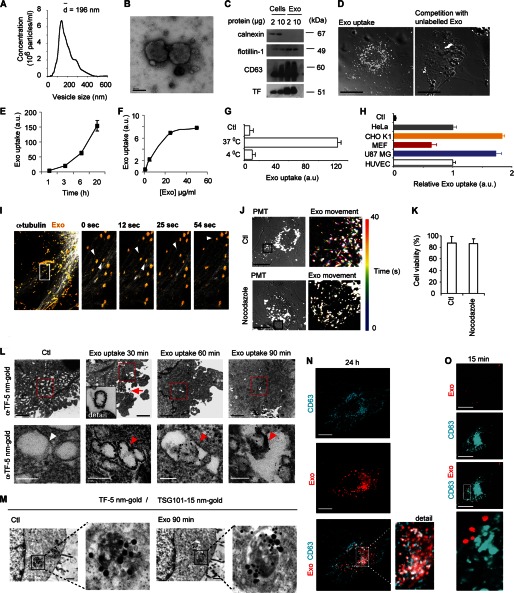
FIGURE 1.
Endocytosis of GBM cell-derived exosome-like extracellular vesicles. A, characterization of U87 MG-derived vesicles by nanoparticle tracking analysis. B, electron microscopy validates intact vesicles. Scale bar, 100 nm. C, immunoblot analysis of cells and exosome-like vesicles for the exosomal markers CD63, TF, and flotillin-1, and the ER marker calnexin. D, confocal microscopy analysis of exosome uptake in the absence or presence of an excess (×4) unlabeled exosomes. Scale bars, 15 μm. E and F, time (E), and concentration (F)-dependent uptake of exosomes using flow cytometry analysis. G, insignificant passive uptake of exosomes at 4 °C. H, exosome uptake in human cervix adenocarcinoma cells (HeLa), chinese hamster ovary cells (CHO K1), mouse embryonic fibroblasts (MEF), U87 MG and HUVEC cells (n = 3). Values are normalized to HUVEC ( = 1). Control (Ctl) represents cells without exosomes. Flow cytometry graphs represent mean fluorescent values of one of two representative experiments generating similar results, error bars are ± S.D.; a.u., arbitrary units. I, exosomes move along microtubule tracks. COS-7 cells were transfected with pIRESneo-EGFP-α-tubulin 24 h prior to the addition of PKH26 labeled exosomes for an additional 16 h. Movie sequences display (4 boxed individual images, 0 s, 12 s, 25 s, 54 s) of exosome transport (yellow) along microtubule (white). Arrowheads depict intracellular, motile exosomes. Images were captured using a C-Apochromat 20X/0.8 M27 objective, 4.0 zoom, pinhole setting of 31 μm and laser gains of 5.5% (561 nm) and 5% (488 nm). Image size was x:512, y:512, and images were captured during 2 min and 11 s. Scale bar, 20 μm. For full-length movie, see supplemental video S2. J, reduced exosome transport in HUVECs treated for 10 min with 10 μg/ml nocodazole (lower panels) compared with Control (no treatment, upper panels). Pictures shown (Exo movement) represent color-coded data from time series of exosome movement in an overlay in which every time point (during 40 s) corresponds to a color. Images were captured using a C-Apochromat 63X/1.20W korr M27 objective (zoom 3.6). Scale bars, 50 μm. For full-length movies, see supplemental videos S3 and S4. K, cell viability is intact as measured by trypan blue exclusion after 10 min of nocodazole (10 μg/ml) treatment. L, electron microscopy images of compartments with internalized tissue factor (TF)-bearing exosomes over time. Low magnification overviews (upper panels, scale bars, 100 μm) confirm the intracellular localization of exosomes, and cropped pictures (lower panels, scale bars, 100 nm) demonstrate intraluminal vesicles (red arrowheads) positive for α-TF 5 nm gold particles. Note that endogenous vesicular structures in HUVECs are negative for TF (lower left panel, white arrowhead). M, electron microscopy colocalization studies in HUVECs of GBM cell-derived exosomes detected by α-TF 5 nm gold particles and MVBs distinguished by anti-TSG101 15 nm gold particles (scale bars, 100 μm). Ctl: no addition of exosomes. N–O, exosomes reside in a CD63-positive compartment after long-term incubation (24 h) but do not colocalize with CD63 at the cell surface. U87MG cells stably expressing CD63-mCherry (N) or transiently transfected with CD63-GFP (O) were incubated with PKH-labeled exosomes for the indicated time periods. Cells were washed in 1 m NaCl and PBS to remove nonspecifically bound exosomes before fixation and confocal microscopy analysis. CD63 (turquoise) and internalized exosomes (red) were captured at the indicated time points. Scale bars, 15 μm.