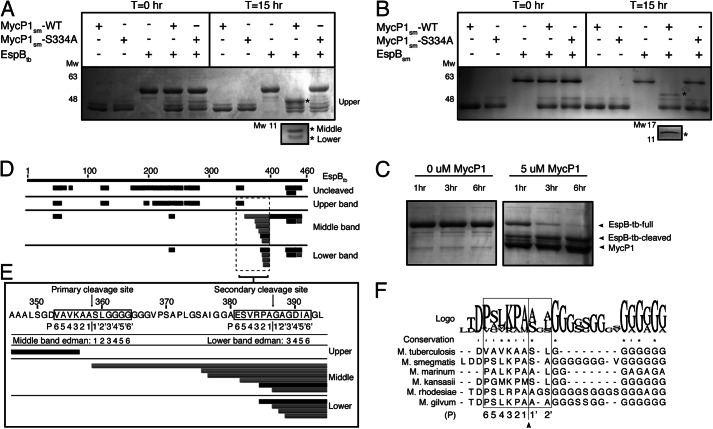
FIGURE 1.
MycP1 cleavage of EspB. A, SDS-PAGE gel showing MycP1 cleavage of EspBtb, with the three EspB cleavage products indicated by asterisks. B, SDS-PAGE gel showing MycP1 cleavage of EspBsm, with two EspB cleavage products indicated by asterisks. C, SDS-PAGE gel showing MycP1-dependent EspB degradation over 6 h. D, LC-MS/MS analysis of uncleaved EspBtb compared with the three EspBtb cleavage products. Tryptic peptides (black) and semitryptic peptides in (gray) are mapped against the full EspB sequence (residues 1–460), indicated by the numbered upper black bar. E, EspBtb sequence surrounding the proposed cleavage sites with LC-ESI-MS/MS, Edman sequencing results, and proposed P residues mapped. F, multiple sequence alignment of EspB homologues with the residues involved in conferring specificity in subtilisin-like enzymes indicated by a box.