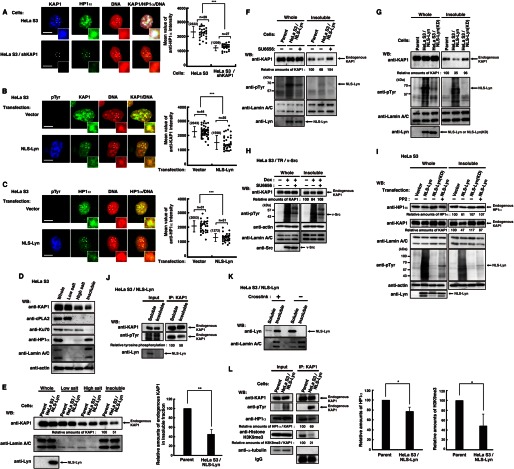
FIGURE 3.
Decrease in the levels of KAP1 association with heterochromatin induced by nuclear tyrosine phosphorylation. A, parental HeLa S3 cells or HeLa S3/shKAP1 cells were extracted, fixed, and triply stained with the indicated antibodies and PI. The plot represents the mean intensity of anti-HP1α staining in each cell. B and C, HeLa S3 cells transfected with vector or NLS-Lyn were cultured for 24 h. Cells were extracted, fixed, and triply stained with the indicated antibodies and PI. The plot represents the mean fluorescence intensity of anti-KAP1 (B) or anti-HP1α (C) staining in each cell. D, HeLa S3 cells were subjected to subcellular fractionation. Immunoblotting (WB) was performed with the indicated antibodies. E, parental HeLa S3 or HeLa S3/NLS-Lyn cells treated with 0.5 mm Na3VO4 were cultured for 1.5 h. Cells were subjected to subcellular fractionation. Immunoblotting was performed with the indicated antibodies. Relative amounts of KAP1 were quantitated. Error bars, S.D. F–I, cells were subjected to subcellular fractionation. Immunoblotting was performed with the indicated antibodies. F, parental HeLa S3 or HeLa S3/NLS-Lyn cells treated with SU6656 or DMSO for 3 h were cultured in the presence of 0.5 mm Na3VO4 during the last 1.5 h. G, parental HeLa S3, HeLa S3/NLS-Lyn, or HeLa S3/NLS-Lyn(KD) cells treated with 0.5 mm Na3VO4 were cultured for 1.5 h. H, HeLa S3/TR/v-Src cells treated with or without doxycycline (Dox) were cultured for 24 h in the presence of DMSO or SU6656 during the last 12 h. I, HeLa S3 cells transfected with the indicated plasmids were cultured for 36 h in the presence or absence of 10 μm PP2 during the last 24 h. J, HeLa S3/NLS-Lyn cells treated with 0.5 mm Na3VO4 were cultured for 1.5 h. Cells were subjected to subcellular fractionation. The insoluble fraction was suspended in high salt buffer and solubilized by sonication. Endogenous KAP1 was immunoprecipitated (IP) with anti-KAP1 antibody from the soluble and the insoluble fractions. Immunoblotting was performed with the indicated antibodies. K, HeLa S3/NLS-Lyn cells were cross-linked in medium containing 1% paraformaldehyde and incubated at 4 °C for 15 min. The cross-linking reaction was stopped by adding glycine to 0.125 m. Cells were subjected to subcellular fractionation. Immunoblotting was performed with the indicated antibodies. L, parental HeLa S3 or HeLa S3/NLS-Lyn cells treated with 0.5 mm Na3VO4 were cultured for 1.5 h and extracted by low salt buffer. The resultant insoluble fraction was suspended in high salt buffer, solubilized by sonication. Endogenous KAP1 was immunoprecipitated with anti-KAP1 antibody. Immunoblotting was performed with the indicated antibodies. Relative amounts of HP1α and histone H3K9me3 were quantitated.