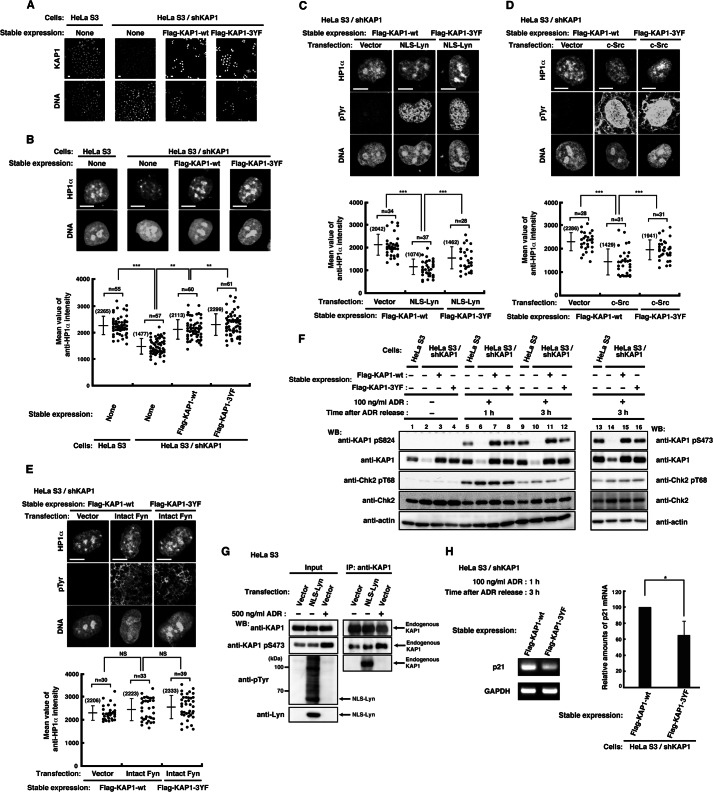
FIGURE 4.
Effect of tyrosine phosphorylation of KAP1 at Tyr-449, Tyr-458, and Tyr-517 on the association of HP1α with chromatin. A, parental HeLa S3 cells or HeLa S3/shKAP1 cells in which endogenous KAP1 had been knocked down and replaced with KAP1-wt or KAP1–3YF were fixed and doubly stained with anti-KAP1 antibody and PI. B, cells were extracted, fixed, and doubly stained with anti-HP1α antibody and PI. The plot represents the mean intensity of anti-HP1α staining in each cell. C–E, HeLa S3/shKAP1 cells expressing KAP1-wt or KAP1–3YF were transfected with the indicated plasmids and cultured for 36 h. Cells were extracted, fixed, and triply stained with the indicated antibodies and TOPRO-3. The plot represents the mean intensity of anti-HP1α staining in each cell. F, HeLa S3/shKAP1 cells expressing KAP1-wt or KAP1–3YF were treated with 100 ng/ml ADR for 1 h and lysed at the indicated times after the removal of ADR. Immunoblotting (WB) was performed with the indicated antibodies. G, HeLa S3 cells transfected with vector or NLS-Lyn were cultured for 24 h. Cells treated with DMSO or 500 ng/ml ADR for 1 h were cultured for 3 h after the removal of ADR or DMSO. Endogenous KAP1 was immunoprecipitated (IP) with anti-KAP1 antibody. Immunoblotting was performed with the indicated antibodies. H, cells were treated as described in F. The levels of p21 expression were assessed by semiquantitative RT-PCR, and the amounts of p21 product were quantitated by measuring band intensities and normalizing to the levels of GAPDH. Error bar, S.D.