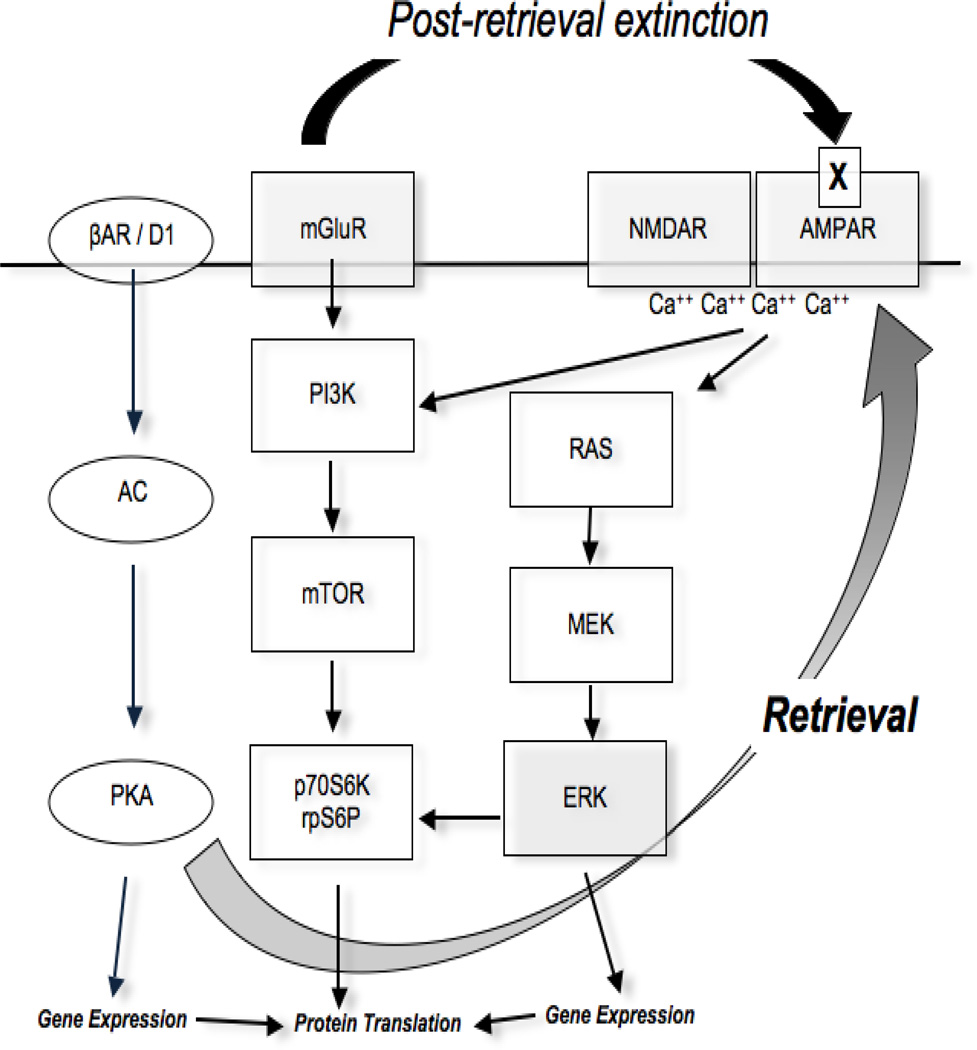
Fig. 1.
Molecular pathways of memory reconsolidation. Molecular signalling cascades downstream of β-adrenergic receptors (βAR), dopamine D1 receptor (D1) and N-methyl-d-aspartate receptors (NMDAR) have been shown to be implicated in reconsolidation. The mammalian target of rapamycin (mTOR), influenced by the activities of neuronal surface receptors and channels including NMDAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR), and dopaminergic and metabotropic glutamate receptors (mGluR), also seems to play a key role in reconsolidation through the phosphorylation of kinase p70S6 (p70S6K) and ribosomal protein S6 (rpS6P). βAR and D1 activate adenylyl cyclase (AC) leading to protein kinase A (PKA) phosphorylation and gene expression. PKA also phosphorylates AMPAR after memory retrieval. Ca++ influx from NMDAR and AMPAR activates the extracellular signal-regulated kinase pathway (ERK), by rat sarcoma (RAS) and mitogen- activated protein kinase (MEK), and phosphatidylinositide 3-kinases (PI3K). Phosphorylation of ERK increases the levels of the transcription factors ets-like gene-1 (Elk-1), phosphorylated cAMP response element binding (pCREB), known to be involved in reconsolidation. PI3K activation leads to mTOR activation resulting in p70S6K and rpS6 phosphorylation and synaptic protein translation. mGluR1 receptor activation is requested after post-retrieval extinction leading to the selective removal of synaptic calcium-permeable AMPAR (X symbol in the Figure). Although the molecular details are not yet known the mGluR1-induced internalization of AMPAR may distinguish reconsolidation update from conventional extinction