Abstract
Objectives
To evaluate the test characteristics of clinical examination (CE) with the addition of bedside emergency ultrasound (CE+EUS) compared to CE alone in determining skin and soft tissue infections (SSTIs) that require drainage in pediatric patients.
Methods
This was a prospective study of CE+EUS as a diagnostic test for the evaluation of patients 2 months to 19 years of age evaluated for SSTIs in a pediatric emergency department (ED). Two physicians clinically and independently evaluated each lesion, and the reliability of the CE for diagnosing lesions requiring drainage was calculated. Trained pediatric emergency physicians (EPs) performed US following their CEs. The authors determined and compared the test characteristics for evaluating a SSTI requiring drainage for CE alone and for CE+EUS for those lesions in which the two EPs agreed and were certain regarding their CE diagnosis (clinically evident). The performance of CE+EUS was evaluated in those lesions in which the two EPs either disagreed or were uncertain of their diagnosis (not clinically evident). The reference standard for determining if a lesion required drainage was defined as pus expressed at the time of the ED visit, or within two days by follow-up assessment.
Results
Three hundred and eighty-seven lesions underwent CE+EUS and were analyzed. CE agreement between physicians was fair (K = 0.38). For the 228 lesions for which physicians agreed and were certain of their diagnoses, sensitivity was 94.7% for CE and 93.1% for CE+EUS (difference −1.7%, 95% CI = −3.4% to 0%). The specificity of CE was 84.2% compared to 81.4% for CE+EUS (difference −2.8%, 95% CI = −9.7% to 4.1%). For lesions not clinically evident based on CE, the sensitivity of CE was 43.7%, compared with 77.6% for CE+EUS (difference 33.9%, 95% CI = 1.2% to 66.6%). The specificity of CE for this group was 42.0%, compared with 61.3% for CE+EUS (difference 19.3%, 95% CI = −13.8% to 52.4%).
Conclusions
For clinically evident lesions, the addition of US did not significantly improve the already highly accurate CE for diagnosing lesions requiring drainage in this study population. However, there were many lesions that were not clinically evident, and in these cases, US may improve the accuracy of the CE.
INTRODUCTION
Skin and soft tissue infections (SSTIs) encompass a spectrum of disease from cellulitis to frank abscess. Treatment of most abscesses requires incision and drainage1–8 or needle aspiration,5 while cellulitis is treated with systemic antibiotics and supportive care. It may be difficult to distinguish between these two entities, as both have similar clinical features.9 In some cases the physical examination alone may provide insufficient information as to the extent of the infection.8 Therefore, as the presence or absence of purulent material may be difficult to determine, children may undergo unnecessary drainage procedures, possibly with exposure to procedural sedation. Conversely, if a fluid collection is missed on examination, the patient may experience worsening of the disease process, and require further treatment and interventions, including additional emergency department (ED) visits.
The ability to differentiate a cellulitis from an abscess by ultrasound (US) has been well described in the radiology literature.10–14 However, many institutions do not have the resources to support soft tissue US performed by technologists and interpreted by radiologists during “off-hours.”15,16 US performed at the patient’s bedside by the clinician offers the opportunity of immediate integration of sonographic and clinical information. Studies involving bedside emergency ultrasound (EUS) have shown that soft tissue US is a useful adjunct to the clinical examination (CE) in adults and may alter clinical management.17,18
The role of EUS in the ED evaluation of SSTIs in the pediatric population has not been fully evaluated. Extrapolating data from the adult to the pediatric population is problematic, as young children may be less cooperative for the US examination than adults, making an examination difficult to accurately perform and interpret. The primary aim of this study was to compare the test characteristics of CE alone, consisting of history and physical examination, to those of CE with the addition of emergency US (CE+EUS) for determining SSTIs requiring drainage in pediatric patients. We hypothesized that test characteristics for CE+EUS would be better than those of CE alone.
METHODS
Study Design
We conducted a prospective evaluation of CE+EUS as a diagnostic test for the evaluation of SSTIs in pediatric ED patients. The study adhered to the Standards for Reporting of Diagnostic Accuracy (STARD) criteria for research.19 Parents or guardians provided written consent, and patients capable of understanding the consent process provided assent. The institutional review board at our institution approved the study.
Study Setting and Population
Patients were enrolled on a convenience basis from July 2008 through April 2010 in a tertiary-care, university-affiliated, urban pediatric ED, with approximately 90,000 visits annually. During the study period, trained research associates screened all ED visits between 7 AM and 12 AM, seven days per week using the ED electronic patient tracking system.
Patients were eligible for enrollment if they were 2 months to 19 years of age and the attending physician responsible for the management of the patient (“treating physician”) considered the diagnosis of an isolated SSTI requiring treatment with systemic antibiotic therapy. It was standard practice at our institution to treat SSTIs with systemic antibiotics, regardless of whether a drainage procedure was performed. We included lesions with a history of drainage prior to the ED visit, since a history of drainage did not preclude the possibility of a recurrent or persistent collection requiring drainage at the ED visit. We excluded patients without an English-speaking parent or guardian available, previously enrolled patients, patients who had imaging (such as US or computed tomography) of the lesion prior to arrival to our ED, patients in custody of law enforcement, and immunocompromised patients. We also excluded lesions involving the face, genital, or perirectal region; post-operative wound infections; paronychia; and infections surrounding indwelling catheters or tubes, because we determined, a priori, that either a subspecialty consultant would likely make management decisions for these lesions, or the infection would represent a process other than a simple SSTI.
Study Protocol
EUS Use
A total of eight pediatric emergency medicine (PEM) attending and fellow physicians (“study physicians”) were trained to perform soft tissue US. The principal investigator served as one of the study physicians and had completed prior US training in accordance with the recommendations of the American College of Emergency Physicians.20 Seven other physicians with limited or no prior experience in EUS completed a six-hour training program that included lectures and hands-on scanning practice.21 Following the course, these physicians were supervised at the bedside by experienced sonologists (senior emergency medicine residents and the principal investigator) and had to perform a minimum of five proctored exams, 80% of which had to be correctly interpreted and deemed technically adequate prior to enrolling patients in the study. This training curriculum has been previously reported, and demonstrated that novice PEM physicians were able to develop technical proficiency in soft tissue EUS and interpret images with excellent agreement with an expert.21 In addition, the expert sonologist, who was blinded to the clinical examination by the study and treating physicians, and to the outcome of the lesion, reviewed 75% of the US scans performed throughout the study and provided feedback to the study physicians for quality assurance.
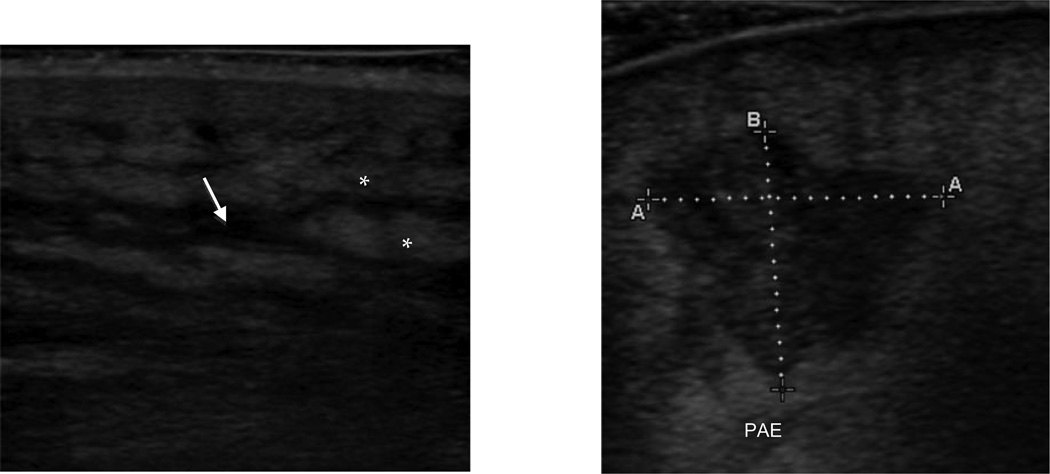
A standardized data collection sheet was used to obtain US information and study physicians recorded digital video clips and still images in a systematic fashion. Study physicians were instructed to scan the area of interest and record video clips in two orthogonal planes, and to note signs of cellulitis (cobblestoning, thickened and/or hyperechoic dermis), and signs of an abscess (hypoechoic, heterogeneously echogenic, irregularly shaped lesion with posterior acoustic enhancement). If a fluid collection was identified, it was measured in three orthogonal axes and still images were recorded. It was at the study physician’s discretion whether to image the contralateral side for comparison, to use compression to evaluate for mobility of pus, or to use color flow Doppler. Figure 1 demonstrates the spectrum of lesions identified by US.
Figure 1.
Ultrasound images demonstrating fat lobules (*) with interlobular fluid (arrow) consistent with cellulitis (left) and irregular-shaped hypoechoic fluid collection (measured) with posterior acoustic enhancement (PAE) consistent with an abscess (right)
Ultrasound examinations were performed using a SonoSite MicroMaxx (SonoSite Inc, Bothell, WA) machine using a 6–13 MHz or 5–10 MHz linear array transducer, or in rare cases of deep buttock abscesses, a 2–5 MHz curved array transducer. The digitally recorded clips (in .avi format) and images (in .bmp format) were transferred from the US machine onto a compact flash disc and then to a computer hard drive for image review.
Enrollment
Two groups of patients were enrolled. The primary group (CE+EUS group) was comprised of those patients who presented when a study physician (who could not also be the treating physician for that patient) was available to perform CE+EUS. Patients who presented when no study physician was available to perform EUS (no-EUS group) were enrolled as a second group in order to evaluate for selection bias. In the no-EUS group, no EUS was performed and we obtained only demographic and lesion information, and final outcome. A physician member of the treating team, using a tape measure, measured the length of erythema, induration, and fluctuance in two dimensions at the bedside for all patients. Up to three separate lesions could be assessed per enrolled patient. In such cases, each lesion was analyzed as a separate data point. If a patient had more than three lesions, the treating physician decided which three should be evaluated.
For the EUS group, treating attending physicians performed CE and recorded “yes,” “no,” or “uncertain” regarding the presence of a lesion requiring drainage. Similarly, study physicians, blinded to the treating physician CE, performed their CEs and recorded their diagnoses. Following each CE, the study physician performed EUS and recorded an opinion (“yes,” “no,” or “uncertain”) as to whether the combined CE+EUS revealed a lesion requiring drainage. In order to minimize test-review bias,22 all treating and study physician CEs and EUS exams were performed prior to diagnostic or treatment procedures by the treating team.
For the very reason that the diagnostic accuracy of EUS in the evaluation of SSTIs in children is unknown, we had clinical equipoise as to its performance, and therefore conducted the study as a non-intervention study, without using the information from CE+EUS in the clinical management of patients. As such, the treating attending physician made all clinical decisions blinded to the results of EUS. Patients and families were also blinded to the study physician interpretation of CE+EUS. Treating physicians were able to order any tests they thought clinically indicated to manage and treat the patients (including US exams from the Department of Radiology); however, the clinical impression was recorded prior to obtaining such ancillary studies. At the time of this study, EUS was not authorized for use in clinical decision-making within our institution’s ED, and its use was strictly limited to this study; therefore, physicians were unable to use US outside the confines of the study protocol.
Data Collection
We collected data for each patient on a study form completed by the research associate, the treating physician caring for the patient, and the study physician performing EUS, and entered the data into an electronic database (Access, Microsoft Corp., Redmond, WA, 2007). After completion of data entry, we verified all data by comparing database entry with all original data collection forms.
Outcomes
Using information from previous studies, we developed an a priori composite reference standard using the findings from the drainage procedure (if performed), or information obtained from follow-up.23,24 Therefore, the outcome of a lesion requiring drainage was defined in one of two ways: 1) drainage procedure in the ED (incision and drainage, needle aspiration, or manual pressure), revealed pus; or 2) follow-up17,23 (if no ED drainage procedure was performed), indicating progression within two calendar days to spontaneous drainage or pus expressed from a drainage procedure.
We recognized there would be multiple methods in which to obtain follow-up information. We, therefore, established a hierarchy, such that in cases with more than one follow-up method available, the method of highest priority (in descending order: medical record review, phone follow-up, primary care physician follow-up questionnaire) was used. Medical record review was placed before other methods of follow-up as it would best capture objective evaluation of lesions by a medical provider. If we were unable to obtain any follow-up information on a lesion, that lesion was excluded from the analysis. Two study investigators completed medical record review and three trained research assistants and the principal investigator performed phone follow-up, all of whom were blinded to study physician assessments (CE and EUS results).
Data Analysis
We described subject and lesion characteristics using standard summary statistics. To evaluate for selection bias, we compared demographics and outcomes between patients and lesions in the EUS and no-EUS groups. For patients in the EUS group, we estimated sensitivity, specificity, positive and negative predictive values, and the associated 95% confidence intervals (CIs) for CE alone and for CE+EUS. Prior studies have shown a lack of agreement between physicians in their clinical evaluations of SSTIs.25,26 Therefore, we further divided the CE+EUS group such that we analyzed clinically evident lesions separately from those which were not clinically evident, based on CE. Clinically evident lesions were defined as those for which both the treating and study physicians agreed and were each certain in their diagnoses for whether the lesion required drainage. Lesions that were not clinically evident were those for which the treating and study physicians either disagreed with each other or were “uncertain” in their diagnoses. We determined interrater reliability between physicians using percent agreement and Cohen’s kappa statistic.27
We used a model-based approach,28 at the level of the lesion, extended to a multilevel mixed-effects logistic regression model,29 to adjust for the multilevel nature of the data. We used the bootstrap method with 1,000 replications30 to obtain valid CIs and to compare the test characteristics of the two diagnostic tests. The bootstrap approach took into account both the non-independence of these paired measurements, and adjusted for clustering of data at the level of the lesion and also the study physician.
Assuming a baseline sensitivity of physician CE similar to that published in a study of adult patients with SSTIs (86%),17 a type 1 error rate of 0.05, and an intraclass correlation coefficient of 0.5 for the relationship of lesions within patients, we estimated that a sample size of 393 lesions would provide 80% power to detect at least a 9% difference in the sensitivity of CE+EUS compared to CE alone. We performed all statistical analysis using Stata 10.0 software (StataCorp, College Station, TX).
RESULTS
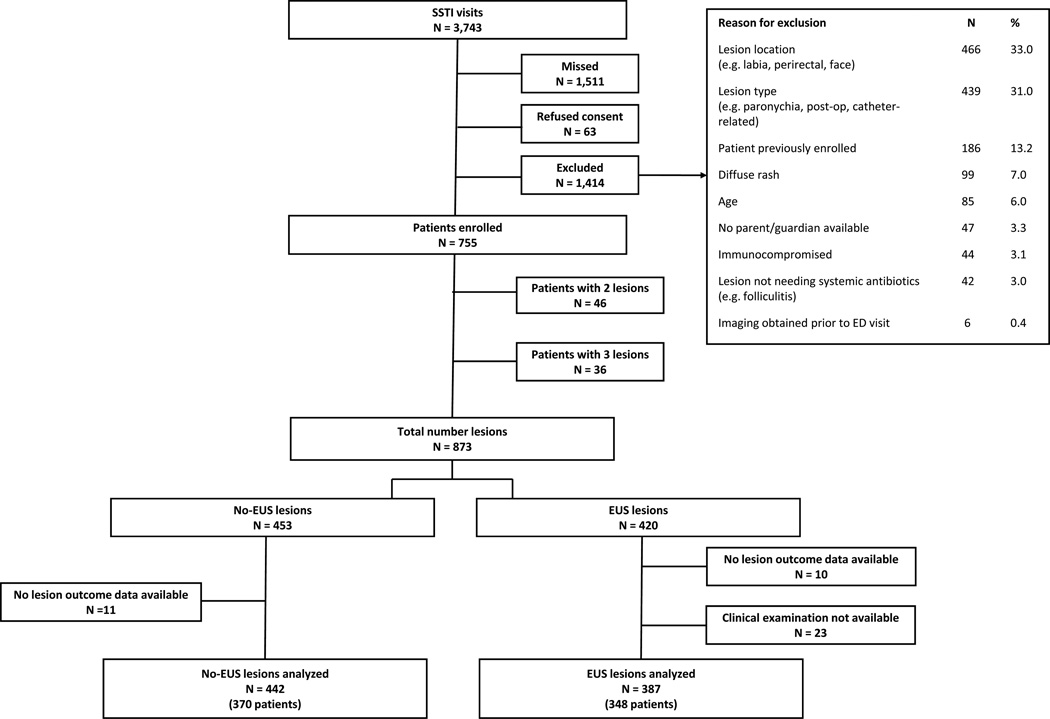
During the study period, 3743 children presented with SSTIs (Figure 2). Of these, 1511 were present when either research associates or study physicians were unavailable. Of the remaining 2232, 63 refused to participate in the study and 1414 patients did not meet inclusion criteria. Four hundred and twenty lesions were enrolled in the EUS group, and 453 lesions were enrolled in the no-EUS group. We obtained outcome data on 98% of lesions in both the EUS and no-EUS groups. Those lesions that did not have follow-up information were excluded from the analysis of test characteristics. An additional 23 lesions in the EUS group were not included in the analysis because either the treating or study physician CE was not available, resulting in 387 lesions in 348 patients to be analyzed in the EUS group, and 442 lesions in 370 patients in the no-EUS group.
Figure 2.
Flow diagram of patient/lesion enrollment
SSTI = skin or soft tissue infection
Patients enrolled in the EUS group had a median age of 7 years (IQR 2.8–14.1 ) and the majority (65%) were African American (Table 1). Most lesions were located on the leg or buttock, and the prevalence of an abscess requiring drainage, as defined by our reference standard, was 62%. In assessing for selection bias for patients enrolled when a study physician was available, patient demographics between the EUS and no-EUS groups were similar, with the exception of a higher proportion of females in the EUS group. Lesions in the EUS group had a larger median area of induration, as measured on CE, when compared with lesions in the no-EUS group, but were otherwise similar.
Table 1.
Comparison of patient demographics and lesion characteristics for all enrolled patients by group
| Characteristics | EUS group | No-EUS group |
P value |
|---|---|---|---|
| Patient | N=348 | N=370 | |
| Median age, yrs (IQR) | 7.0 (1.8–14.1) | 5.8 (2.1–12.8) | 0.60 |
| Race/ethnicity | 0.64 | ||
| African American, non-Hispanic | 225 (65) | 248 (67) | |
| White, non-Hispanic | 91 (26) | 96 (26) | |
| Hispanic | 14(4) | 9 (2) | |
| Female | 201 (58) | 182 (49) | 0.02 |
| SSTI exposure history* | 144 (41) | 145 (39) | 0.5 |
| Lesion | N=387 | N=442 | |
| Location | 0.96 | ||
| Leg | 122 (32) | 150 (34) | |
| Buttock | 96 (25) | 105 (24) | |
| Arm | 26 (7) | 34 (8) | |
| Abdomen | 24 (6) | 33 (7) | |
| Pus noted prior to ED visit | 193 (50) | 220 (50) | 0.88 |
| Median area, cm2 (IQR) | |||
| Erythema | 9 (1–30) | 6 (1–20) | 0.14 |
| Induration | 6 (1–16) | 4 (1–12) | 0.001 |
| Fluctuance | 0 (0– 2) | 0 (0– 1) | 0.52 |
| Lesions not drained in ED | 156 (40) | 174 (39) | 0.78 |
| Lesion outcome determined by medical record review | 78 (50) | 89 (51) | |
| Lesion outcome determined by phone follow-up | 78 (50) | 85 (49) | |
| Lesion outcome determined by PCP questionnaire | 0 (0) | 0 (0) | |
| Prevalence of abscess requiring drainage (per reference standard of either ED drainage or follow-up) | 239 (62) | 266 (60) | 0.64 |
Exposure history defined as personal or close contact with history of SSTI within last year Data are reported as n (%) unless otherwise noted.
SSTI = skin and soft tissue infection; PCP = primary care physician
Table 2 shows study physician CE and CE+EUS impressions, and lesion outcomes for all 387 lesions in the EUS group. Overall, the addition of a positive EUS result to an already positive CE did not result in a significant improvement in the proportion of correctly diagnosed lesions with pus (83.2% vs 87.6%; difference 4.5%, 95% CI = −2.9% to 11.7%). Similarly, the addition of a negative EUS result to an already negative CE did not result in a statistically significant improvement in the proportion of lesions correctly diagnosed as not requiring drainage (difference 9.1%, 95% CI = −2.4% to 19.7%).
Table 2.
Study physician CE and CE+EUS impressions and outcome (lesion requiring drainage) for all lesions in the EUS group
| Physician Impression | CE Positive (n=196) 50.5% |
CE Negative (n=106) 27.3% |
CE Uncertain (n=85) 22.0% |
|---|---|---|---|
| ED drainage procedure | 161 (82.1%) | 19 (17.9%) | 51 (60.0%) |
| Lesions requiring drainage* | 163 (83.2%) | 24 (22.6%) | 52 (61.8%) |
| CE+EUS positive | n=170 | n=11 | n=49 |
| Lesions requiring drainage* | 149 (87.6%) | 6 (54.6%) | 38 (77.6%) |
| CE+US negative | n=14 | n=81 | n=27 |
| Lesions requiring drainage* | 5 (35.7%) | 11 (13.6%) | 11 (40.7%) |
| CE+US uncertain | n=12 | n=14 | n=9 |
| Lesions requiring drainage* | 9 (75.0%) | 7 (50.0%) | 3 (33.3%) |
N = 387
Outcome of lesion requiring drainage defined as pus obtained during ED drainage procedure or on follow-up.
CE = clinical exam; CE+EUS = clinical exam with emergency bedside ultrasound
The agreement between the CE assessments of the study physicians and the treating physicians was 62.8% (kappa 0.38, 95% CI = 0.30 to 0.46, indicating only “fair” agreement).31 Table 3 compares the test characteristics for the study physician CE and CE+EUS for the clinically evident lesions and shows no improvement with the addition of EUS for those lesions. For the remaining 159 lesions (41.1%) that were not clinically evident, CE+EUS was positive in 83, negative in 55, and uncertain in 21 lesions. In the 138 lesions when CE+EUS was either positive or negative, CE+EUS had a sensitivity of 77.6% (95% CI = 58.2% to 89.7%) and a specificity of 61.3% (95% CI = 45.6% to 71.2%), for an overall accuracy of 70.3% (95% CI = 61.9% to 77.8%).
Table 3.
Test characteristics of study physician clinical examination (CE) alone and clinical examination plus emergency ultrasound (CE+EUS) of those lesions that were clinically evident and not clinically evident
| N | Lesions requiring drainage per outcome |
Sensitivity, % (95% CI) |
Specificity, % (95% CI) |
PPV (95 % CI) |
NPV (95% CI) |
|
|---|---|---|---|---|---|---|
| Clinically evident (n=228) | ||||||
| Study/treating MD CE+ | 156 | 144 | 94.7 (90.2 to 97.9) |
84.2 (74.7 to 91.7) |
92.3 (87.3 to 96.3) |
88.9 (80.2 to 95.3) |
| Study/treating MD CE− | 72 | 8 | ||||
| Study MD CE+EUS + | 147 | 134 | 93.1 (88.4 to 96.6) |
81.4 (70.9 to 90.0) |
91.2 (85.8 to 95.4) |
85.1 (76.1 to 92.5) |
| Study MD CE+EUS − | 67 | 10 | ||||
| Study MD CE+EUS uncertain | 14 | 8 | ||||
| Difference |
−1.7 (−3.4 to 0) |
−2.8 (−9.7 to 3.2) |
−1.2 (−3.8 to 1.7) |
−3.8 (−7.2 to 0.2) |
||
| Not clinically evident (n=159) | ||||||
| Study MD CE+ | 40 | 19 | 43.7 (0.0 to 85.3) |
42.0 (0.0 to 79.5) |
42.9 (0.0 to 66.7) |
45.6 (0.0 to 71.4) |
| Study MD CE− | 34 | 16 | ||||
| Study MD CE uncertain | 85 | 52 | ||||
| Study MD CE+EUS + | 83 | 59 | 77.6 (58.2 to 89.7) |
61.3 (45.6 to 71.2) |
71.1 (62.5 to 79.5) |
69.1 (56.5 to 79.1) |
| Study MD CE+EUS − | 55 | 17 | ||||
| Study MD CE+EUS uncertain | 21 | 11 | ||||
| Difference |
33.9 (1.2 to 66.6) |
19.3 (−13.8 to 52.4) |
28.2 (8.8 to 47.6) |
23.2 (0.6 to 45.8) |
We examined further the 159 lesions that were considered not clinically evident. The median age of the patients in this group was 4.7 years (IQR 1.6 to 12.0 years), compared to 9.3 years (IQR 1.9 to 15.2 years) in the clinically evident group (p = 0.003). Fifty (31.5%) lesions were located on the leg, and 44 (27.7%) were located on the buttock, which was similar to the location distribution in the clinically evident group (p = 0.14). Seventy-three (45.9%) were noted to have pus prior to the ED visit, which was similar to that in the clinically evident group (p = 0.27). Eighty-seven lesions (54.7%) were abscesses requiring drainage per the outcome criteria, compared to 66.7% requiring drainage in the clinically evident group (p = 0.02).
The median abscess volume as measured on EUS for those lesions without either expression of pus by ED drainage or by follow-up criteria was 0.24 cm3 (IQR: 0.06 cm3 to 0.64 cm3), compared to a median volume of 0.51 cm3 (IQR 0.16 cm3 to 1.39 cm3) for lesions that did yield pus (p < 0.001).
Seventy-two lesions in the EUS group also underwent US evaluation in the Department of Radiology as requested by the treating physician. For 10 of these lesions, the study physicians rated their CE+EUS examination as uncertain. Evaluating the remaining 62 lesions, study physician CE+EUS had a higher sensitivity (80%, 95% CI = 58.6% to 92.3%) for detecting a lesion requiring drainage than radiology US (45.7%, 95% CI = 34.3% to 60.5%; difference 34.2%, 95% CI = 16.7% to 54.5%). The difference in specificity between the two groups was not significant.
Three-hundred and three US exams were reviewed by the expert sonologist for the presence of abscesses. The agreement between the study physician and expert as expressed by the kappa statistic was 0.69 (95% CI = 0.64 to 0.70), indicating “substantial” agreement.31
DISCUSSION
Our study of pediatric patients demonstrates that for clinically evident lesions, CE was highly accurate for diagnosing SSTIs requiring drainage and the addition of EUS did not improve the accuracy. In cases that were not clinically evident, we found that CE+EUS was reasonably sensitive, and more sensitive than for CE, although specificity was only moderate and not statistically improved from CE alone. Our findings differ from those of prior studies. One study of 135 adult patients with SSTIs17 found that EUS had a sensitivity of 98%, and a specificity of 88%. Nearly half (25 out of 51) of the patients who did not receive drainage (presumed cellulitis by EUS) were lost to follow-up and were excluded from the analysis. If any of these lesions ultimately expressed pus, the sensitivity of EUS would have been significantly lower. There have been two small studies of pediatric patients with SSTIs,32,33 in which CE+EUS sensitivity was shown to be 90% to 98% compared with CE sensitivity of 75% to 79%, and CE+EUS specificity was shown to be 69% to 83% compared with CE specificity of 67% to 80%. These studies suffered from small sample sizes,32,33 as well as a lack of patient follow-up.33 None of the aforementioned studies involved formal statistical comparisons in order to assess for true differences between the CE and CE+EUS. For those studies that included follow-up, there was a lengthy follow-up period, which may not have reflected the disease pathology occurring at the time of the ED visit when CE+EUS was performed. We restricted our follow-up period to two days in order to minimize the misclassification of lesions. Perhaps the most important methodological difference is that in these prior studies, physicians were allowed to incorporate EUS into lesion management decisions. This practice may have led to a bias in favor of the CE+EUS test characteristics. SSTIs represent a spectrum of disease, and this spectrum is well visualized by US. Consequently, there may be differences of opinion as to which lesions would be amenable to drainage versus conservative therapy. Therefore, although a lesion produced an amount of purulent drainage when incision and drainage was employed due to EUS information, the lesion may have also responded to systemic antibiotics alone if no drainage had been performed. Conversely, if the conclusion after EUS was that a lesion did not require drainage, it is possible that if the lesion had been drained in the ED, pus would have been obtained.
Our study was able to capture the inherent physician uncertainty that exists when diagnosing SSTIs. In addition, it is important to note that there was only “fair” agreement between physicians in their diagnostic clinical impressions, which is similar to findings by other investigators.25 As a result, physicians should take into account the limitations of CE in diagnosing SSTIs.
As expected, our study did demonstrate a larger sonographic abscess volume in the group of lesions requiring drainage; however, further investigation is needed into sonographic measurements that define the need for a drainage procedure.
Although our study only enrolled patients when a study physician was available, based on our analysis of the EUS and no-EUS groups, no clinically relevant selection bias was present. Our follow-up rate was high, with only 2% of lesions without outcome data; therefore, verification bias22 was unlikely to affect our results. Finally, the study physicians’ US scans were reviewed throughout the study to ensure adequate performance of EUS. Analysis of the reliability of those images indicated that study physicians were proficient in their interpretation of images.
The sensitivity and negative predictive value of CE+EUS were significantly higher than those of radiology-performed US. This may indicate an advantage of sonologists to directly integrate the CE in their interpretation of imaging modalities that outweighs the experience and knowledge of imaging specialists. This information may be useful to practitioners who, due to uncertainty of a clinical diagnosis of a lesion requiring drainage, are considering a radiology-performed US.
LIMITATIONS
The limitations of this study should be noted. Our reference standard was designed to identify lesions requiring drainage by incorporating ED management and a narrow follow-up period, since it would have been unethical to require ED drainage of all lesions, particularly those deemed to not require drainage by the treating team. It is possible that this standard lead to misclassification in several ways. First, lesions correctly identified as not requiring drainage at the time of EUS might have progressed to abscess formation within the two-day follow-up period, leading to misclassification as a false negative due to the natural progression of disease; and in fact, the lesion would not have benefitted from drainage at the time of the EUS. Also, there is the case where EUS was interpreted as a lesion not requiring drainage because the lesion was sonographically small, but the lesion was drained and any amount of pus was obtained. This may have represented a false negative misclassification of a lesion that might have been adequately treated by antibiotics alone. Currently, there is no consensus as to how small a collection of pus does not require drainage, and how much pus drained from a lesion constitutes evidence of a collection that should have been drained. Finally, some abscesses identified by EUS may have been inadequately drained (i.e. no pus with drainage), resulting in the misclassification of EUS as a “false positive.” All of these scenarios would have biased our study results toward the null hypothesis of no difference between the test characteristics of CE+EUS and CE. These issues will only be resolved when sonographic findings and their relationship with the spectrum of interventions (conservative management vs. drainage) have been more extensively studied, allowing us to better define the “cut-offs” for invasive procedures by developing characteristics of lesions that do and do not benefit from drainage based on clinical outcomes.
Lesion management was determined by the treating physician, and therefore was likely biased in favor of clinical impression. However, if treating physicians had been able to incorporate EUS results, lesion management may have been different and the study physician CE and CE+EUS test characteristics may have been higher. However, as stated in the Methods, we determined blinding of the EUS results to be the most methodologically rigorous means of evaluating our hypotheses, given the current state of scientific knowledge on this topic and the resultant clinical equipoise regarding the diagnostic accuracy of EUS.
Despite blinding the study physicians to the treatment team management plan, there may have been test review bias if subtle clues revealed the management plan to the study physician prior to performing the US (e.g. an intravenous catheter in place suggesting sedation, or topical anesthesia over the lesion suggesting drainage procedure to be done). This bias would also minimize any difference in test characteristics between CE and CE+EUS.
Finally, our study may not be generalizable to non-PEM trained physicians, those without significant exposure to SSTIs, or those with more prior experience using EUS. The prevalence of an abscess requiring drainage per our reference standard was 62%; therefore, our predictive values are only applicable to those institutions with similar prevalences.
CONCLUSIONS
We demonstrated that in our pediatric study population, the addition of emergency ultrasound to the clinical examination for the diagnosis of skin and soft tissue infections does not improve on the test characteristics of the clinical examination alone for clinically evident lesions. Many lesions are not clinically evident, and in these cases emergency ultrasound may be useful as a diagnostic adjunct. Further trials are needed that incorporate emergency ultrasound into patient care, and evaluate whether the addition of emergency ultrasound improves clinical outcomes in pediatric patients with skin and soft tissue infections.
Acknowledgements
The authors thank the study physicians (Elizabeth Alpern, Monika Goyal, Toni Gross, Rakesh Mistry, Sage Myers, Kyle Nelson, and Mark Zonfrillo) who donated their time and skills in order to enroll patients, to Stephen Yakscoe and Matthew Albert for their dedication to the study as research coordinators, and to the patients and families who volunteered to participate in this study. We are also grateful to SonoSite, Inc. for the use of their ultrasound machine for the duration of the study and to the decision editor of Academic Emergency Medicine for his insightful suggestions.
Funding: Funding was provided by the National Institutes of Health, National Institute of Allergy and Infectious Diseases (F32AI080063), and by the Nicholas Crognale Chair for Emergency Medicine, The Children’s Hospital of Philadelphia, Philadelphia, PA.
Footnotes
Prior presentations: This study was presented at the annual meeting of the Pediatric Academic Societies, Denver CO, May 2011.
Disclosures: Dr. Marin received support in the form of loaned ultrasound equipment for the duration of the study from SonoSite, Inc.
References
- 1.Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357:380–390. doi: 10.1056/NEJMcp070747. [DOI] [PubMed] [Google Scholar]
- 2.Buescher ES. Community-acquired methicillin-resistant Staphylococcus aureus in pediatrics. Curr Opin Pediatr. 2005;17:67–70. doi: 10.1097/01.mop.0000147906.30720.4d. [DOI] [PubMed] [Google Scholar]
- 3.Frank AL, Marcinak JF, Mangat PD, Schreckenberger PC. Community-acquired and clindamycin-susceptible methicillin-resistant Staphylococcus aureus in children. Pediatr Infect Dis J. 1999;18:993–1000. doi: 10.1097/00006454-199911000-00012. [DOI] [PubMed] [Google Scholar]
- 4.McCaig LF, McDonald LC, Mandal S, Jernigan DB. Staphylococcus aureus-associated skin and soft tissue infections in ambulatory care. Emerg Infect Dis. 2006;12:1715–1723. doi: 10.3201/eid1211.060190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitch MT, Manthey DE, McGinnis HD, Nicks BA, Pariyadath M. Videos in clinical medicine. Abscess incision and drainage. N Engl J Med. 2007;357:e20. doi: 10.1056/NEJMvcm071319. [DOI] [PubMed] [Google Scholar]
- 6.Lee MC, Rios AM, Aten MF, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23:123–127. doi: 10.1097/01.inf.0000109288.06912.21. [DOI] [PubMed] [Google Scholar]
- 7.Llera JL, Levy RC. Treatment of cutaneous abscess: a double-blind clinical study. Ann Emerg Med. 1985;14:15–19. doi: 10.1016/s0196-0644(85)80727-7. [DOI] [PubMed] [Google Scholar]
- 8.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–1406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 9.Swartz MN. Clinical practice. Cellulitis. N Engl J Med. 2004;350:904–912. doi: 10.1056/NEJMcp031807. [DOI] [PubMed] [Google Scholar]
- 10.Arslan H, Sakarya ME, Bozkurt M, Unal O, Dilek ON, Harman M. The role of power Doppler sonography in the evaluation of superficial soft tissue abscesses. Eur J Ultrasound. 1998;8:101–106. doi: 10.1016/s0929-8266(98)00065-2. [DOI] [PubMed] [Google Scholar]
- 11.Braunstein EM, Silver TM, Martel W, Jaffe M. Ultrasonographic diagnosis of extremity masses. Skeletal Radiol. 1981;6:157–163. doi: 10.1007/BF00347181. [DOI] [PubMed] [Google Scholar]
- 12.Chao HC, Lin SJ, Huang YC, Lin TY. Sonographic evaluation of cellulitis in children. J Ultrasound Med. 2000;19:743–749. doi: 10.7863/jum.2000.19.11.743. [DOI] [PubMed] [Google Scholar]
- 13.Fornage BD. Soft-tissue masses. Clin Diagn Ultrasound. 1995;30:21–42. [PubMed] [Google Scholar]
- 14.Latifi HR, Siegel MJ. Color Doppler flow imaging of pediatric soft tissue masses. J Ultrasound Med. 1994;13:165–169. doi: 10.7863/jum.1994.13.3.165. [DOI] [PubMed] [Google Scholar]
- 15.Desser TS, Rubin DL, Schraedley-Desmond P. Coverage of emergency after-hours ultrasound cases: survey of practices at U.S. teaching hospitals. Acad Radiol. 2006;13:249–253. doi: 10.1016/j.acra.2005.09.091. [DOI] [PubMed] [Google Scholar]
- 16.Heller M, Crocco T, Patterson J, Prestosh J, Krall J, Hill RG. Emergency ultrasound services as perceived by directors of radiology and emergency departments. Am J Emerg Med. 1995;13:430–431. doi: 10.1016/0735-6757(95)90132-9. [DOI] [PubMed] [Google Scholar]
- 17.Squire BT, Fox JC, Anderson C. ABSCESS: applied bedside sonography for convenient evaluation of superficial soft tissue infections. Acad Emerg Med. 2005;12:601–606. doi: 10.1197/j.aem.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Tayal VS, Hasan N, Norton HJ, Tomaszewski CA. The effect of soft-tissue ultrasound on the management of cellulitis in the emergency department. Acad Emerg Med. 2006;13:384–388. doi: 10.1197/j.aem.2005.11.074. [DOI] [PubMed] [Google Scholar]
- 19.Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ. 2003;326:41–44. doi: 10.1136/bmj.326.7379.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American College of Emergency Physicians. [Accessed Mar 10, 2013];Policy Statement: Emergency Ultrasound Guidelines. 2008 Available at www.acep.org/workarea/downloadasset.aspx?id=32878.
- 21.Marin JR, Alpern ER, Panebianco NL, Dean AJ. Assessment of a training curriculum for emergency ultrasound for pediatric soft tissue infections. Acad Emerg Med. 2011;18:174–182. doi: 10.1111/j.1553-2712.2010.00990.x. [DOI] [PubMed] [Google Scholar]
- 22.Begg CB, McNeil BJ. Assessment of radiologic tests: control of bias and other design considerations. Radiology. 1988;167:565–569. doi: 10.1148/radiology.167.2.3357976. [DOI] [PubMed] [Google Scholar]
- 23.Jaeschke R, Guyatt G, Sackett DL. Users' guides to the medical literature. III. How to use an article about a diagnostic test. A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA. 1994;271:389–391. doi: 10.1001/jama.271.5.389. [DOI] [PubMed] [Google Scholar]
- 24.Knottnerus JA, Muris JW. Assessment of the accuracy of diagnostic tests: the cross-sectional study. J Clin Epidemiol. 2003;56:1118–1128. doi: 10.1016/s0895-4356(03)00206-3. [DOI] [PubMed] [Google Scholar]
- 25.Giovanni JE, Dowd MD, Kennedy C, Michael JG. Interexaminer agreement in physical examination for children with suspected soft tissue abscesses. Pediatr Emerg Care. 2011;27:475–478. doi: 10.1097/PEC.0b013e31821d8545. [DOI] [PubMed] [Google Scholar]
- 26.Marin JR, Bilker W, Lautenbach E, Alpern ER. Reliability of clinical examinations for pediatric skin and soft-tissue infections. Pediatrics. 2010;126:925–930. doi: 10.1542/peds.2010-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 28.Coughlin SS, Trock B, Criqui MH, Pickle LW, Browner D, Tefft MC. The logistic modeling of sensitivity, specificity, and predictive value of a diagnostic test. J Clin Epidemiol. 1992;45:1–7. doi: 10.1016/0895-4356(92)90180-u. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein H. Multilevel statistical models. 3rd ed. London, UK: Hodder Arnold; 2003. [Google Scholar]
- 30.Stata Corp. Stata Reference Manual Resease 7. College Station, TX: Stata Corp; 2001. bstrap:bootstrap sampling and estimation; pp. 164–174. [Google Scholar]
- 31.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 32.Sivitz AB, Lam SH, Ramirez-Schrempp D, Valente JH, Nagdev AD. Effect of bedside ultrasound on management of pediatric soft-tissue infection. J Emerg Med. 2010;39:637–643. doi: 10.1016/j.jemermed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Iverson K, Haritos D, Thomas R, Kannikeswaran N. The effect of bedside ultrasound on diagnosis and management of soft tissue infections in a pediatric ED. Am J Emerg Med. 2012;30(8):1347–1351. doi: 10.1016/j.ajem.2011.09.020. [DOI] [PubMed] [Google Scholar]