Abstract
OBJECTIVE
To report our experience of reduced-dose argatroban in a patient with suspected heparin-induced thrombocytopenia (HIT) and Child-Pugh class C liver disease and review the relevant literature to summarize current recommendations on argatroban use in patients with severe liver disease.
CASE SUMMARY
A 58-year-old male with Child-Pugh class C liver disease (Model for End-Stage Liver Disease [MELD] score = 31, total bilirubin 4.5 mg/dL) and hemodialysis-dependent renal failure was hospitalized with acute deep vein thrombosis (DVT). Three days after heparin initiation for DVT, he developed thrombocytopenia. Given his heparin exposure (both for treatment of DVT and ongoing hemodialysis), HIT was suspected and all heparinoids were immediately discontinued. Argatroban was initiated for the treatment of HIT while laboratory testing for HIT antibodies and the serotonin release assay were completed. Because of the patient’s advanced liver disease, the starting dose of argatroban was reduced to 0.2 µg/kg/min, with frequent monitoring of the activated partial thromboplastin time (aPTT) (goal 60–85 seconds). Despite this dose reduction, the aPTT was supratherapeutic. Following further dose reductions, a final argatroban maintenance dose of 0.05 µg/kg/min was necessary for the attainment of goal aPTTs.
DISCUSSION
Reducing the starting dose of argatroban to 0.5 µg/kg/min is recommended in patients with liver disease. Nevertheless, this recommended dose is largely based on data from patients with more moderate liver disease (eg, Child-Pugh class A or B), and dosing in more advanced liver disease remains largely unexplored. Patients with more advanced liver disease may require additional dose reductions to avoid supratherapeutic concentrations of anticoagulation agents and to minimize bleeding risk.
CONCLUSIONS
This report illustrates the importance of careful selection of argatroban dosing and appropriate aPTT monitoring in patients with severe liver disease. Excessive anticoagulation may precipitate major bleeding complications, placing patients with this complicated disease at undue risk.
Keywords: argatroban, deep vein thrombosis, heparin-induced thrombocytopenia, liver disease, platelet, renal failure
Heparin-induced thrombocytopenia (HIT) is an immune-mediated form of thrombocytopenia occurring in 1–5% of patients exposed to heparinoids.1 HIT develops when antibodies of the IgG (or less commonly IgM or IgA) class react against complexes of heparin and platelet factor 4 and bind to platelet membrane receptors.2,3 This binding leads to platelet activation with release of procoagulant factors, activation of other cells, and a resulting hypercoagulable state.
Clinically, HIT is characterized by thrombocytopenia that develops 5–10 days following initial heparin exposure (classic HIT) or within 24 hours of heparin reexposure (rapid-onset HIT). Thrombocytopenia in HIT, defined as a 50% or more decrease relative to the baseline platelet count, reaches an average nadir of 50,000–60,000/µL.4–6 If left untreated, up to 50% of patients with HIT will develop arterial or venous thrombosis, including deep vein thrombosis (DVT), stroke, arterial occlusion leading to limb loss, and pulmonary embolism.4,6
HIT is a clinical pathologic diagnosis. Estimating the pretest probability (PTP), using the 4Ts (thrombocytopenia, timing of thrombocytopenia relative to heparin, thrombosis, and etiology; Table 1), and then combining this PTP with the results of laboratory testing (eg, HIT antibody testing) improve diagnostic accuracy.4,5,7,8 When HIT is suspected, all heparin products should be immediately discontinued and non–heparin-based anticoagulants (direct thrombin inhibitors [DTIs]) initiated to reduce the risk of venous and arterial thromboembolism.5,6 Argatroban is one of several Food and Drug Administration–approved DTIs used in the treatment of HIT.5 Argatroban binds reversibly to the active site of thrombin and inhibits fibrin formation, procoagulant factors, and platelet activation. Argatroban undergoes predominantly hepatic metabolism, catalyzed by the isoenzymes CYP3A4 and CYP3A5 through hydroxylation and aromatization of the 3-methyltetrahydroquinoline ring in the liver.9,10
Table 1.
| The 4Ts | Points | ||
|---|---|---|---|
| 2 | 1 | 0 | |
| Thrombocytopenia | >50% fall or nadir of 20–100 × 109 cells/L | 30–50% fall or nadir 10–19 × 109 cells/L | <30% fall or nadir <10 × 109 cells/L |
| Timing of thrombocytopenia (relative to heparin use) |
5–10 days or <1 day of heparin reexposure within 30 days |
Beyond day 10 or unclear | <5 days No reexposure |
| Thrombosis | Proven (eg, skin necrosis, thrombosis) | Recurrent or suspected | None |
| Etiology of thrombocytopenia | No other cause evident | Possible | Definite |
Scoring: 0–3 = low probability; 4–5 = moderate probability; 6–8 = high probability.
The starting argatroban dose studied in clinical trials for treatment of HIT was 2 µg/kg/min (administered as a continuous intravenous infusion), with frequent laboratory monitoring to achieve an activated partial thromboplastin time (aPTT) of 1.5–3 times the patient’s baseline aPTT. The clearance of argatroban is decreased approximately 4-fold and the elimination half-life is increased from approximately 51 minutes to 181 minutes in patients with hepatic impairment.9 Published data and recommendations from the argatroban package insert suggest reducing the starting dose from 2 µg/kg/min to 0.5 µg/kg/min in patients with moderate hepatic impairment.9,11 This guidance may be one reason that argatroban has been studied and used extensively in patients with HIT and hepatic impairment.
We describe a case demonstrating that applying currently recommended argatroban dose reductions in patients with advanced liver disease may result in excessive anticoagulation. We also review the current literature to summarize argatroban dosing reductions in this challenging condition.
Case Report
A 58-year-old male with alcoholic cirrhosis had end-stage renal disease requiring hemodialysis 3 times per week. When heparin was administered, he developed acute left upper extremity swelling near the dialysis access site. At the time of presentation, the patient’s total bilirubin was 4.5 mg/dL, albumin 1.7 g/dL, alanine aminotransferase 47 U/L, aspartate aminotransferase 101 U/L, aPTT 41 seconds, and international normalized ratio (INR) 1.7. This mild coagulopathy was stable and likely a reflection of underlying liver disease. The patient had mild ascites and no encephalopathy, correlating with a Child-Pugh Score of 11 (class C) and a Model for End-Stage Liver Disease (MELD) score of 31. The MELD and Child-Pugh scoring systems are used to estimate the severity of liver disease (and mortality risk) among patients. The Child-Pugh class has been more commonly used to estimate surgical risk while the MELD is a tool to estimate disease severity for transplant status.12–14 Although imperfect, these scoring systems remain clinically important tools used routinely for patient management.
This patient had a history of chronic, mild thrombocytopenia (baseline platelet count 110 × 103/µL) that was attributed to cirrhosis. He reported no swelling in the right upper extremity or asymmetric swelling of the lower extremities. There was no evidence of symptomatic pulmonary embolism or arterial thrombosis. He had no history of venous thromboses.
On physical examination, the patient was afebrile and hemodynamically stable. Examination of the left upper extremity demonstrated substantial swelling throughout the upper arm and forearm. There were no other acute medical processes or new organ dysfunction. There had been no recent changes to his medications, which included propranolol, rifaximin, and lactulose. A duplex venous ultrasound demonstrated a filling defect in the subclavian vein consistent with acute DVT.
Treatment with intravenous unfractionated heparin (UFH) was started on hospital day 1 (Figure 1). In accordance with institutional protocols, a bolus dose of UFH 5000 units followed by a starting dose of 15 units/kg/h was given and the aPTT was monitored frequently. Warfarin 5 mg daily was also started, initiated on hospital day 1, with daily monitoring of the INR. The patient’s outpatient medications were continued. Because the patient had been exposed to heparin during hemodialysis, the platelet count was monitored daily. Three days after anticoagulation with UFH was initiated, his platelet count had decreased to 49 × 103/µL (Figure 2).
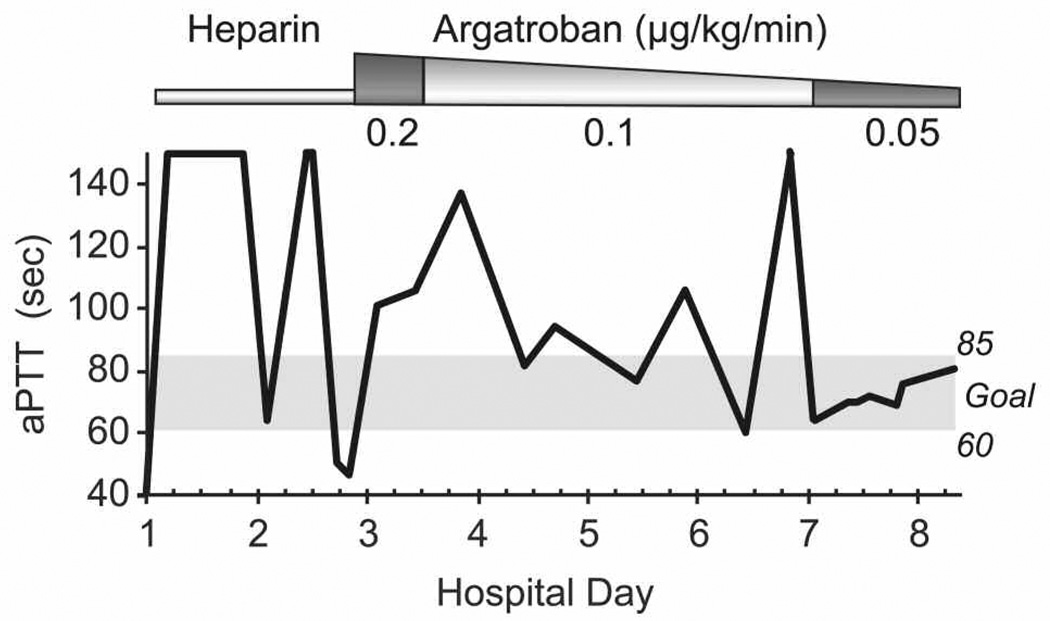
Figure 1.
Levels of activated partial thromboplastin time (aPTT) during the course of anticoagulation. Reduction of the dose to argatroban 0.05 µg/kg/min resulted in therapeutic levels of anticoagulation, defined as an aPTT between 60 and 85 seconds, according to institutional protocols (anticoagulation started on hospital day 1).
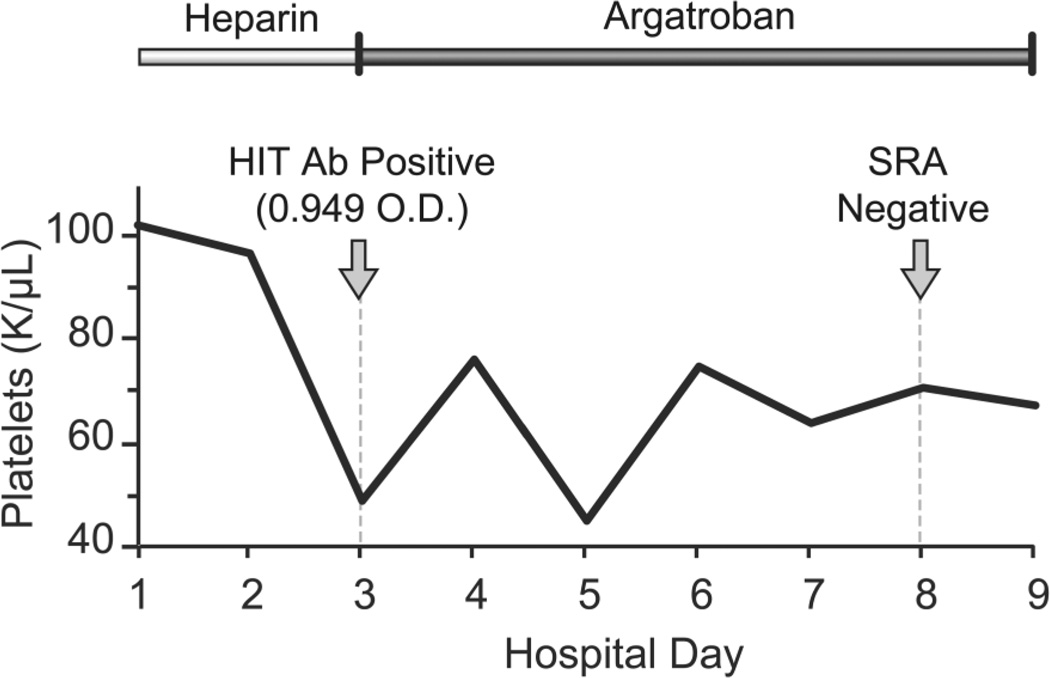
Figure 2.
Timeline of development of thrombocytopenia with resultant testing for HIT in relation to anticoagulation treatment (anticoagulation started on hospital day 1). HIT = heparin-induced thrombocytopenia; O.D. = optical density; SRA = serotonin release assay.
Given the patient’s heparin exposure, including during dialysis on the day prior to admission, HIT was suspected. All heparin products, including heparin flushes, and warfarin were immediately discontinued. Laboratory testing demonstrated an INR of 1.9 and the aPTT was 47 seconds. Systematic anticoagulation due to warfarin therapy was not reversed. Using the 4Ts (Table 1) to determine the likelihood of HIT, we assigned 2 points for thrombocytopenia, 2 points for new thrombosis, 1 point for timing, and 1 point for possible other cause (eg, cirrhosis). The resultant score of 6 is consistent with a high pretest probability for HIT.5,7,8 Therefore, argatroban was initiated while additional evaluation was undertaken by testing for the presence of HIT antibodies and sending plasma for the serotonin release assay.
The starting dose of argatroban was reduced to 0.2 µg/kg/min, given the patient’s severe hepatic impairment and after consideration of published data.10,11,15–19 The aPTT was monitored frequently, with a goal of 60–85 seconds, slightly lower than the recommended goal of 1.5–3 times the baseline aPTT, with values not to exceed 100 seconds. The lower goal for this patient reflects our institution’s anticoagulation safety committee recommendation, which was made with the goal of providing consistency in dosing across a variety of conditions and hopefully decreasing bleeding complications. Nevertheless, despite the dose reduction, the aPTT measured 6 hours following the initiation of argatroban was supratherapeutic, at 101 seconds (Figure 1). Following institutional protocols, the argatroban infusion was stopped and the aPTT was measured every 2–4 hours. The aPTT remained consistently supratherapeutic for more than 24 hours (estimated Naranjo probability scale score indicated a highly probable relationship), perhaps due in part to the patient’s hepatic dysfunction and mild underlying coagulopathy (baseline aPTT 41 seconds).20 Once the aPTT had decreased to within the therapeutic range, argatroban was restarted at a lower infusion rate of 0.1 µg/kg/min (50% reduction). Despite this dose reduction, subsequent aPTTs were almost universally supratherapeutic (Figure 1, range 60–150 seconds). The argatroban dose was then further lowered to 0.05 µg/kg/min. This final dose resulted in appropriate and consistent therapeutic anticoagulation, with all subsequent aPTT values in the therapeutic range (Figure 1).
Clinically, the patient remained stable, with decreased swelling in the upper extremity where the DVT had occurred and no further thrombosis or bleeding. His HIT antibody was positive at 0.949 optical density (OD) units (negative value <0.399) on hospital day 3. The serotonin release assay returned negative results on hospital day 8 (Figure 2). Following this negative result, which excluded HIT, argatroban was discontinued and treatment was transitioned back to UFH. The platelet count remained low, between 45×103/µL and 76×103/µL, but eventually stabilized (Figure 2). The patient continued to improve clinically, with no further change in his platelet count, and warfarin was resumed.
Discussion
DTIs, including lepirudin, bivalirudin, and argatroban, remain the cornerstone of treatment for patients with suspected or confirmed HIT. These parenteral agents reduce the risk of thrombosis in patients with suspected or confirmed HIT. Each of these DTIs is effective in the treatment of patients with HIT, although differences in pharmacology may lead to the selective use of an individual agent in some patients. For example, although argatroban undergoes hepatic metabolism, with a 4-fold decrease in clearance and a 3-fold increase in elimination half-life (eg, from ~51 to 181 minutes), package insert recommendations provide guidance on dose reductions (eg, reducing the starting dose from 2 µg/kg/min to 0.5 µg/kg/min for patients with moderate hepatic impairment).9
Published retrospective studies and observational reports support the need for reduced doses of argatroban in patients with hepatic impairment. For example, Levine and colleagues retrospectively studied 82 hospitalized patients treated with argatroban for HIT, many of whom also had hepatic dysfunction (average estimated MELD = 21).11 More than 50% of these patients had combined hepatic and renal dysfunction. Patients with elevated total bilirubin levels required 50% lower argatroban doses compared with patients with normal bilirubin levels (0.8 µg/kg/min vs 1.6 µg/kg/min), regardless of renal function. Furthermore, argatroban dose requirements correlated inversely with total bilirubin levels up to 5 mg/dL but were unaffected by the presence or absence of renal dysfunction. In these authors’ best-fit equations, for every 1-mg/dL increase in total bilirubin, argatroban dose requirements decreased by 0.38 µg/kg/min.
Critical illness and organ failure, including hepatic dysfunction, also affect argatroban dosing requirements. In a retrospective analysis of 65 critically ill patients, Begelman and colleagues found that argatroban dosing requirements decreased as the number of failing organs increased.18 Beiderlinden and colleagues prospectively studied 24 consecutive patients with multiple organ dysfunction, including liver failure, and suspected HIT who were treated with argatroban. Initial starting doses of argatroban 2 µg/kg/min resulted in supratherapeutic levels of anticoagulation and bleeding complications. When these authors reduced the starting dose to 0.2 µg/kg/min, however, therapeutic levels of anticoagulation were achieved without bleeding complications. 21 In a retrospective study of 12 critically ill patients, argatroban dose requirements were lower in patients with acute hepatic dysfunction (n = 4, individual Child-Pugh/MELD scores not reported) compared with those without hepatic dysfunction (0.10 ± 0.06 vs 0.31 ± 0.14 µg/kg/min).17 Similarly, in 4 patients in the intensive care unit, with MELD scores ranging from 24 to 32 and estimated Child-Pugh class B liver disease, the lowest argatroban dose used was 0.125 µg/kg/min, a dose more than 2-fold higher than our patient’s final dose requirement.22
These studies highlight the importance of appropriate argatroban dosing in patients with either acute or chronic hepatic impairment. Our report supports and extends these findings. Although argatroban dose reductions to 0.5 µg/kg/min in patients with moderate hepatic impairment (with a further reduction to 0.2 µg/kg/min in critical illness) are recommended, this dosing may be too high in patients with severe hepatic impairment, as illustrated by the patient described in this report. To our knowledge, there is only 1 published report of a patient with a similar severity of liver disease treated with argatroban, although the liver injury in that case was a result of inadvertent ligation of the hepatic artery and resulted in ongoing hepatic injury. In that patient’s case, the final argatroban infusion rate for achievement of a therapeutic aPTT was less than 0.06 µg/kg/min, a dose similar to that in our case.23
Our patient also had dialysis-dependent renal failure. Although the excretion of argatroban is primarily through the feces, the half-life of the drug is increased in patients with renal insufficiency and there is a correlation between estimated creatinine clearance and argatroban dose.19,24,25 Nevertheless, while this correlation is not clinically significant and dose adjustments are not routinely recommended in patients with renal failure, we cannot exclude the influence of renal failure on our patient’s final argatroban dose requirements.
These reports highlight the importance of carefully selecting the appropriate dose of argatroban in patients with severe hepatic impairment. Although the starting argatroban dose in our patient was reduced 60%, from 0.5 µg/kg/min to 0.2 µg/kg/min because of advanced liver disease, even this dose was excessive and led to supratherapeutic aPTTs. Two additional dose reductions were required before a stable, therapeutic aPTT was achieved. The final argatroban dose in our patient was 0.05 µg/kg/min, a dose one tenth of that currently recommended as the starting dose for patients with moderate hepatic dysfunction.
Clinicians should carefully consider the initial argatroban dose, as well as subsequent dose titrations, in patients with severe hepatic impairment. In these patients, the currently recommended initial argatroban dose of 0.5 µg/kg/min for moderate hepatic impairment may be too high, leading to excessive anticoagulation and increased risk of bleeding.
Acknowledgments
This work was supported by the National Institutes of Health (grants K23HL092161 and AG040631). We thank Dr. Sara Vazquez of the University of Utah Health Care Thrombosis Center for her critical review of the manuscript, Ms. Diana Lim for her help in figure preparation, and Ms. Brittany Patterson for her editorial assistance.
Footnotes
Conflict of interest: Authors reported none
Contributor Information
Peter M Yarbrough, Department of Internal Medicine, University of Utah, Salt Lake City.
Amir Varedi, Department of Internal Medicine, University of Utah.
Amanda Walker, Department of Internal Medicine, University of Utah.
Matthew T Rondina, Department of Internal Medicine, University of Utah; University Healthcare Thrombosis Service, Salt Lake City.
References
- 1.Warkentin TE, Sheppard JL, Horsewood P, et al. Impact of the patient population on the risk for heparin-induced thrombocytopenia. Blood. 2000;96:1703–1708. [PubMed] [Google Scholar]
- 2.Kelton JG. The pathophysiology of heparin-induced thrombocytopenia: biological basis for treatment. Chest. 2005;127:9S–20S. doi: 10.1378/chest.127.2_suppl.9S. [DOI] [PubMed] [Google Scholar]
- 3.Rauova L, Zhai L, Kowalska MA, et al. Role of platelet surface PF4 antigenic complexes in heparin-induced thrombocytopenia pathogenesis: diagnostic and therapeutic implications. Blood. 2006;107:2346–2353. doi: 10.1182/blood-2005-08-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shantsila E, Lip GY, Chong BH. Heparin-induced thrombocytopenia. A contemporary clinical approach to diagnosis and management. Chest. 2009;135:1651–1664. doi: 10.1378/chest.08-2830. [DOI] [PubMed] [Google Scholar]
- 5.Warkentin TE. How I diagnose and manage HIT. Hematology Am Soc Hematol Educ Program. 2011;2011:143–149. doi: 10.1182/asheducation-2011.1.143. [DOI] [PubMed] [Google Scholar]
- 6.Arepally GM, Ortel TL. Clinical practice. Heparin-induced thrombocytopenia. N Engl J Med. 2006;355:809–817. doi: 10.1056/NEJMcp052967. [DOI] [PubMed] [Google Scholar]
- 7.Lo GK, Juhl D, Warkentin TE, et al. Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4:759–765. doi: 10.1111/j.1538-7836.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 8.Warkentin TE, Heddle NM. Laboratory diagnosis of immune heparin-induced thrombocytopenia. Curr Hematol Rep. 2003;2:148–157. [PubMed] [Google Scholar]
- 9.Argatroban. Research Triangle Park, NC: Glaxo-SmithKline; 2012. Apr, Product information. [Google Scholar]
- 10.Swan SK, Hursting MJ. The pharmacokinetics and pharmacodynamics of argatroban: effects of age, gender, and hepatic or renal dysfunction. Pharmacotherapy. 2000;20:318–329. doi: 10.1592/phco.20.4.318.34881. [DOI] [PubMed] [Google Scholar]
- 11.Levine RL, Hursting MJ, McCollum D. Argatroban therapy in heparininduced thrombocytopenia with hepatic dysfunction. Chest. 2006;129:1167–1175. doi: 10.1378/chest.129.5.1167. [DOI] [PubMed] [Google Scholar]
- 12.Cholongitas E, Papatheodoridis GV, Vangeli M, et al. Systematic review: the model for end-stage liver disease—should it replace Child-Pugh’s classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther. 2005;22:1079–1089. doi: 10.1111/j.1365-2036.2005.02691.x. [DOI] [PubMed] [Google Scholar]
- 13.Causey MW, Steele SR, Farris Z, et al. An assessment of different scoring systems in cirrhotic patients undergoing nontransplant surgery. Am J Surg. 2012;203:589–593. doi: 10.1016/j.amjsurg.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Cholongitas E, Marelli L, Shusang V, et al. A systematic review of the performance of the model for end-stage liver disease (MELD) in the setting of liver transplantation. Liver Transpl. 2006;12:1049–1061. doi: 10.1002/lt.20824. [DOI] [PubMed] [Google Scholar]
- 15.Bates D, Griffin S, Angel B. Clinical experience with argatroban for heparin-induced thrombocytopenia in a large teaching hospital. Can J Hosp Pharm. 2009;62:290–297. doi: 10.4212/cjhp.v62i4.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis BE, Wallis DE, Berkowitz SD, et al. Argatroban anticoagulant therapy in patients with heparin-induced thrombocytopenia. Circulation. 2001;103:1838–1843. doi: 10.1161/01.cir.103.14.1838. [DOI] [PubMed] [Google Scholar]
- 17.Saugel B, Phillip V, Moessmer G, et al. Argatroban therapy for heparin-induced thrombocytopenia in ICU patients with multiple organ dysfunction syndrome: a retrospective study. Crit Care. 2010;14:R90. doi: 10.1186/cc9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Begelman SM, Baghdasarian SB, Singh IM, et al. Argatroban anticoagulation in intensive care patients: effects of heart failure and multiple organ system failure. J Intensive Care Med. 2008;23:313–320. doi: 10.1177/0885066608321246. [DOI] [PubMed] [Google Scholar]
- 19.Hursting MJ, Murray PT. Argatroban anticoagulation in renal dysfunction: a literature analysis. Nephron Clin Pract. 2008;109:c80–c94. doi: 10.1159/000139993. [DOI] [PubMed] [Google Scholar]
- 20.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 21.Beiderlinden M, Treschan TA, Görlinger K, et al. Argatroban anticoagulation in critically ill patients. Ann Pharmacother. 2007;41:749–754. doi: 10.1345/aph.1H569. [DOI] [PubMed] [Google Scholar]
- 22.Williamson DR, Boulanger I, Tardif M, et al. Argatroban dosing in intensive care patients with acute renal failure and liver dysfunction. Pharmacotherapy. 2004;24:409–414. doi: 10.1592/phco.24.4.409.33168. [DOI] [PubMed] [Google Scholar]
- 23.Brand PA, Egberts JH, Scholz J, et al. Argatroban therapy in patients with hepatic and renal impairment. Eur J Anaesthesiol. 2008;25:344–346. doi: 10.1017/S0265021507002839. [DOI] [PubMed] [Google Scholar]
- 24.Arpino PA, Hallisey RK. Effect of renal function on the pharmacodynamics of argatroban. Ann Pharmacother. 2004;38:25–29. doi: 10.1345/aph.1D163. [DOI] [PubMed] [Google Scholar]
- 25.Guzzi LM, McCollum DA, Hursting MJ. Effect of renal function on argatroban therapy in heparin-induced thrombocytopenia. J Thromb Thrombolysis. 2006;22:169–176. doi: 10.1007/s11239-006-9019-2. [DOI] [PubMed] [Google Scholar]