Abstract
Acute thrombocytopenia occurs commonly in hospitalized patients. For most, the etiology of an acutely declining platelet count is obvious and includes sepsis with disseminated intravascular coagulation, large-volume crystalloid infusion, or the administration of cytotoxic therapies, such as chemotherapeutic agents. For others, however, the etiology may be less apparent. In these cases, drug-induced thrombocytopenia (DIT), including heparin-induced thrombocytopenia (HIT), must be a diagnostic consideration. The approach to the hospitalized patient with thrombocytopenia, without an obvious cause, includes a careful medication history to identify potential culprits, such as glycoprotein IIb/IIIa inhibitors, vancomycin, linezolid, β-lactam antibiotics, quinine, antiepileptic drugs, or heparin/low-molecular-weight heparin. Usually, discontinuation of the offending medication is all that is necessary for resolution of thrombocytopenia. Heparin-induced thrombocytopenia, however, is an exception to this general rule given its unique pathogenesis and propensity for thrombotic complications and death. Differentiating between HIT and DIT due to nonheparin medications may prove challenging. Through a careful clinical assessment, consideration of the pre-test probability for HIT, and the thoughtful application of laboratory testing, HIT can be accurately diagnosed. Because patients with HIT have a high risk of thrombosis and bleeding is uncommon, the prompt initiation of an alternative anticoagulant (eg, a direct thrombin inhibitor) is warranted in these patients.
Keywords: drug-induced thrombocytopenia, heparin-induced thrombocytopenia, thrombocytopenia, thrombosis, platelet, heparin
Introduction
The development of acute thrombocytopenia presents a common and potentially catastrophic complication requiring a high degree of clinical acumen for the hospital-based clinician. Defined as an absolute platelet count < 150 000/μL or a 50% decline in platelet count from the patient's baseline platelet count, acute thrombocytopenia has been associated with poor patient outcomes in hospitalized patients.1,2
In hospitalized patients, acute thrombocytopenia is often due to easily recognized clinical circumstances, such as the dilutional effects of large-volume transfusions or crystalloid infusion, increased peripheral platelet destruction from sepsis or disseminated intravascular coagulation (DIC), mechanical destruction related to cardiopulmonary bypass or ventricular assist device, decreased bone marrow production from chemotherapy administration, or platelet sequestration, such as hypersplenism, in those with portal hypertension (Table 1).3 For others, when the etiology of an acutely declining platelet count is not evident, medications must be considered as a potential culprit.4
Table 1. Potential Etiologies of Acute Thrombocytopenia in Hospitalized Patients.
| General Category | Example |
|---|---|
| Spurious | Laboratory error due to platelet clumping |
| Dilutional | Large-volume transfusion or crystalloid infusion |
| Sequestration | Hypersplenism |
| Marrow suppression | Chemotherapy, alcohol |
| Peripheral destruction | Sepsis, DIC, TTP/HUS, post-cardiac surgery, VAD |
| Drug-induced | Penicillin, sulfonamide, linezolid |
| Heparin-induced | HIT |
Abbreviations: DIC, disseminated intravascular coagulation; HIT, heparin-induced thrombocytopenia; HUS, hemolytic uremic syndrome; TTP, thrombotic thrombocytopenic purpura; VAD, ventricular assist device.
Although most patients with drug-induced thrombocytopenia (DIT) have an increased risk of bleeding, those with DIT due to heparin, or heparin-induced thrombocytopenia (HIT), have a low risk of bleeding but, paradoxically, an increased risk of thrombotic complications.3 Distinguishing between HIT and DIT can be challenging, especially with the commonness of thrombocytopenia in hospitalized patients and the ubiquitous use of heparin in the hospital setting.
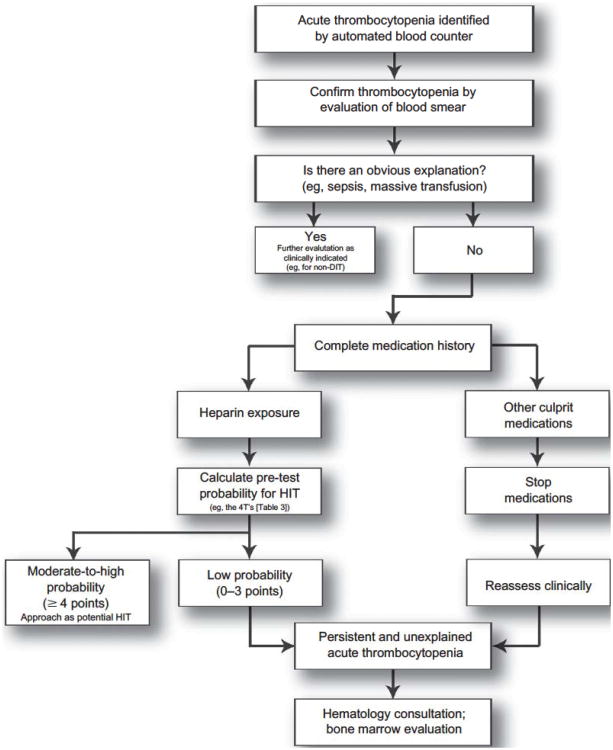
In the Complications After Thrombocytopenia Caused by Heparin (CATCH) registry, acute thrombocytopenia developed in 36.4% of patients who were exposed to heparin for ≥ 96 hours and was associated with an increased risk of death (odds ratio, 3.4; 95% confidence interval, 2.1–5.6).1 Similarly, Laber and Martin5 demonstrated that out of 674 consecutive hospitalized patients exposed to heparin, acute thrombocytopenia developed in 110 (16%), and although 77 (70%) were due to easily recognizable causes, 3 (2.7%) were due to heparin and 14 (12.7%) were due to other medications. Because the prognosis and management of patients with HIT and DIT are very different, quickly and accurately making a diagnosis is paramount. The purpose of this article is to highlight the underlying pathogenesis and associated clinical characteristics of both typical DIT and HIT so that clinicians can rationally approach this common clinical scenario (Figure 1).
Figure 1. A general diagnostic approach to treatment of acute thrombocytopenia in the hospitalized patient.
Abbreviations: DIT, drug-induced thrombocytopenia; HIT, heparin-induced thrombocytopenia.
Common Pharmacologic Agents Implicated in DIT and an Overview of Mechanisms
Over 300 drags have been implicated in DIT6 and the annual incidence of DIT, excluding those cases associated with heparin, is approximately 10 cases per 1 000 000 population.7 Unfortunately, patient-specific risk factors for DIT, other than exposure to certain classes of medications, have not been identified.7
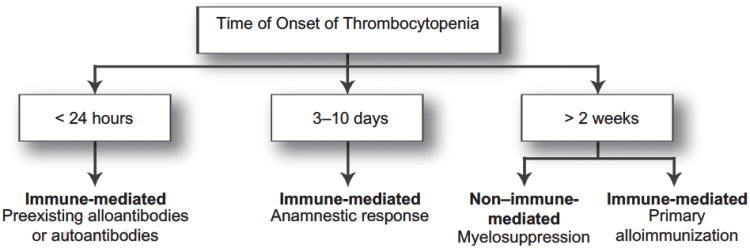
Most drugs causing thrombocytopenia appear to do so through immune-mediated mechanisms: hapten-dependent, drug-glycoprotein complex, ligand-induced binding site, drug-specific antibody, autoantibody, and immune complex. The typical time from initiation of a drug to the onset of immune-mediated DIT is 1 to 2 weeks, although this may vary from hours to years in individual patients. The large heterogeneity in timing of thrombocytopenia development is due to several factors, including whether the patient has been exposed to the drug previously and thus already has circulating, preexisting antibodies (Figure 2). An anamnestic response can lead to the development of DIT in < 24 hours of exposure; in contrast, with primary alloimmunization, 2 to 3 weeks are required for generation of the antibodies and development of DIT.8
Figure 2. Categorization of drug-induced thrombocytopenia by time of onset of thrombocytopenia.
Adapted from Arch pathol Lab Med.8
In contrast, non–immune-mediated DIT occurs through bone marrow suppression, usually from antineoplastic medications (alkylating agents, antimetabolites, and cytotoxic drugs), although several antiviral agents, tolbutamide, and thiazide diuretics have also been implicated.8,9 Because the mechanism of non–immune-mediated DIT is one of myelosuppression, the decrease in platelets is typically a dose-dependent response. The platelet count can begin to decline within several days, but nadir platelet counts occur in ≥ 2 weeks, as that is the time required to consume the preexisting platelet pool in the setting of drug-related megakaryocyte depletion.
In hospitalized patients, although numerous medications have been implicated in DIT (Table 2), there are a few commonly administered drugs that deserve particular mention: glycoprotein IIb/IIIa antagonists, antibiotics, quinine/quinidine, and certain antiepileptic medications. Glycoprotein IIb/IIIa receptor antagonists, a class of antithrombotic drugs, are a common cause of DIT and can induce life-threatening thrombocytopenia. Occurring in 1% to 2% of patients treated with abciximab and 0.2% to 1% of patients treated with eptifibatide or tirofiban, thrombocytopenia can develop within a few hours of first drug exposure (due to preexisting antibodies), and is often severe (platelet count < 20 000/μL).10–12 These preexisting antibodies can be formed either when a patient has been previously exposed to an offending agent or may be due to naturally occurring antibodies.13 Importantly, an anamnestic response occurs in 10% to 12% of patients and may lead to severe DIT on reexposure.14 Therefore, caution and close laboratory monitoring are prudent in these patients, both upon initial or repeat exposure.
Table 2. Drugs Associated with Thrombocytopenia.
| Observational Study Evidence | ||
|---|---|---|
| Carbamazepine | ||
| Phenobarbital | ||
| Phenytoin | ||
| Valproic acid | ||
|
| ||
|
Case Report Evidence (probable or definite causality rating) | ||
|
| ||
| Abciximab | Felbamate | Nitroglycerin |
| Acetaminophen | Fluconazole | Octreotide |
| Acyclovir | Gold salts | Oxacillin |
| Albendazole | Haloperidol | Para-aminosalicyiic acid |
| Aminoglutethimide | Heparin | Penicillamine |
| Aminosalicylic acid | Hydrochlorothiazide | Pentoxifylline |
| Amiodarone | Ibuprofen | Piperacillin |
| Amphotericin B | Inamrinone | Prednisone |
| Ampicillin | Indinavir | Primidone |
| Aspirin | Indomethacin | Procainamide |
| Atorvastatin | Interferon alfa | Pyrazinamide |
| Captopril | Isoniazid | Quinidine |
| Cephalosporins | Isotretinoin | Quinine |
| Chlorothiazide | Itraconazole | Ranitidine |
| Chlorpromazine | Levofloxacin | Recombinant hepatitis B vaccine |
| Chlorpropamide | Levamisole | Rifampin |
| Cimetidine | Linezolid | Simvastatin |
| Ciprofloxacin | Lithium | Sirolimus |
| Clarithromycin | Lotrafiban | Sulfasalazine |
| Clopidogrel | Low-molecular-weight heparins |
Sulfonamides |
| Danazol | Measles, mumps, and rubella vaccines |
Sulindac |
| Deferoxamine | Meclofenamate | Suramin |
| Diazepam | Mesalamine | Tamoxifen |
| Diazoxide | Methyldopa | Tirofiban |
| Diclofenac | Minoxidil | Tolmetin |
| Diethylstilbestrol | Morphine | Trimethoprim |
| Digoxin | Nalidixic acid | Vancomycin |
| Ethambutol | Naproxen | |
Adapted from Arch Pathol Lab Med.8
Antibiotics are another common cause of DIT, although the mechanism differs among various agents. Vancomycin-induced thrombocytopenia is thought to occur through a hapten-drug mechanism because vancomycin binds to platelet glycoproteins, leading to the generation of antibodies that bind to the platelet surface and cause cell lysis.15 In a study of 34 patients with platelet-reactive antibodies to vancomycin, thrombocytopenia developed within an average of 8.4 days after vancomycin initiation; however, some patients developed thrombocytopenia as early as 24 hours and as late as 27 days. In comparison, DIT can also occur with administration of the newer oxazolidinone antibiotic, linezolid, although the mechanism is not clearly understood. The incidence of DIT with linezolid has been reported as 2.9% to 64.7%16,17 and is higher in patients with decreased renal function (defined as a glomerular filtration rate < 50 mL/min).18 Like vancomycin or linezolid, penicillins, cephalosporins, and trimethoprim-sulfamethoxazole may also cause DIT.19
Quinine and quinidine are well documented to cause DIT. Although less commonly used, these drugs may still be encountered as treatment for nocturnal leg cramps and can be found in tonic beverages. Thrombocytopenia can occur during initial exposure to these drugs, although most cases normally require 7 to 10 days of ongoing exposure, during which time antibodies are formed.
Other medications, including the commonly prescribed antiepileptic drugs phenytoin and carbamazepine, interact noncovalently with platelet membranes and form antibodies, resulting in platelet lysis.8,19 The antibody binds to the drug-glycoprotein complex only in the presence of a soluble drug.
Clinical Features of DIT
Most patients with DIT will have received the offending medication for ≥ 1 week before thrombocytopenia develops, due to the time required for the development of antibodies. However, patients given tirofiban, abciximab, and eptifibatide may develop DIT within hours of the initial exposure, as naturally occurring antibodies to these drugs are present.19 Once antibodies are present (or if they are naturally occurring), patients may have symptoms of presyncope, fever, nausea, chills, and even hypotension with associated loss of consciousness (especially with high-titer antibodies).20 Microangiopathic hemolytic anemia and renal failure (eg, thrombotic thrombocytopenic purpura syndrome) may be seen with quinine.21
The thrombocytopenia in DIT is usually moderate to severe (nadir platelet count < 20 000/μL) but can occasionally be as low as 1000/μL. As expected, the risk of bleeding is related to the degree of thrombocytopenia. The clinical features of HIT will be described below, although it is important to highlight that the thrombocytopenia in DIT is usually more severe than in HIT.
Minor bleeding complications (including epistaxis, petechiae, and bruising) have been reported in approximately two-thirds of patients with DIT, while the rate of more serious bleeding, including gastrointestinal hemorrhage, is < 10%.8 In cases where the platelet count is < 10000/μL, extensive purpuric lesions of skin and mucosal surfaces, including gastrointestinal (wet purpura), are not uncommon. Catastrophic bleeding, such as intracranial hemorrhage, has been reported but is rare.22 Once the offending agent has been stopped, most bleeding complications resolve within several days and platelet counts recover in 4 to 8 days. Rarely, the thrombocytopenia may persist for several weeks.
Diagnosis and Management of DIT
In acutely ill hospitalized patients, the diagnosis of DIT can be challenging. Other potential causes of the thrombocytopenia, including idiopathic thrombocytopenic purpura (FTP), DIC, and sepsis, should be considered in the differential diagnosis and appropriate laboratory testing undertaken. Evaluation of the peripheral blood smear may be necessary and can help the hospitalist eliminate spurious platelet clumping as a possibility, and distinguish between bone marrow suppression and peripheral platelet destruction, including microangiopathic etiologies. After initial assessment and review of the peripheral blood, a hematology consultation for evaluation and consideration of bone marrow biopsy, with assessment of marrow aplasia, myelodysplasia, or a neoplastic process, may be prudent, especially in cases of slowly resolving DIT or cases in which diagnosis remains uncertain.
If there are no obvious causes of thrombocytopenia, a careful history of drug exposure, including specific questions about quinine exposure (eg, consumption of tonic water),23 should be undertaken and possible offending agents discontinued. Importantly, DIT is largely a clinical diagnosis as there are no readily available specific laboratory methods for the diagnosis of DIT, although some specialized centers may have the ability to more easily perform these tests.19
With the discontinuation of the offending medication, the platelet count usually returns to normal within 4 to 8 days, although, rarely, the thrombocytopenia may persist for > 2 weeks.19 During recovery, the platelet count should be monitored to verify resolution of thrombocytopenia and the patient should be carefully followed for signs of bleeding. Allergy to the offending medication should be well documented in the medical record and the patient should be advised to avoid the offending medication in the future. Further, when a drug is confirmed as the etiology of thrombocytopenia, clinicians should report the episode to the US Food and Drug Administration (FDA) Adverse Event Reporting System at www.fda.gov/medwatch.
Aside from stopping an offending medication, the optimal management of patients with DIT has received little attention. Patients with thrombocytopenia and hemorrhage can generally be supported with cessation of the offending agent and platelet transfusion, and for those with a severe and prolonged clinical course, the coadministration of intravenous immune globulin or plasma exchange may be beneficial.24,25 Corticosteroids are of uncertain benefit in DIT but are often recommended because ITP cannot usually be definitively excluded as an alternative diagnosis. If given, steroids should be stopped as soon as platelet count recovery has occurred. If thrombocytopenia recurs with cessation, DIT is less likely and other causes, specifically ITP, are probable.6 Additionally, although thrombopoietic agents have been shown to be helpful in patients with refractory ITP, their role in DIT has not been established.26
Heparin-lnduced Thrombocytopenia
Heparin-induced thrombocytopenia is a common form of DIT that has received increased attention among hospitalist and critical care physicians due to its relative frequency and potential risk of limb- or life-threatening thromboses. Although, classically, there are 2 forms of HIT, their mechanisms, clinical presentation, and management differ significantly. Non–immune-mediated HIT, referred to as heparin-associated thrombocytopenia or HIT type I, is common, occurring in up to 30% of patients exposed to heparinoids—usually within the first few days of heparin administration27—and is not associated with thrombosis or bleeding. The thrombocytopenia, which is usually mild (platelet count > 100 000/μL), gradually resolves within several days, without interruption of heparin administration.28,29
Heparin administration may also result in the more clinically relevant immune-based thrombocytopenia, formally termed HIT type II but now simply referred to as HIT, which is characterized by a more severe thrombocytopenia that typically develops 5 to 10 days after heparin exposure or within hours to days of reexposure. Heparin-induced thrombocytopenia, although less common than heparin-associated thrombocytopenia, is associated with a high thrombotic risk that, if uninterrupted, may lead to catastrophic complications such as limb loss or death.
Epidemiology of HIT
Despite the potentially catastrophic nature of HIT and the ubiquitous use of heparin in hospitals, clinicians often fail to consider HIT as a diagnostic possibility in the hospitalized patient with a declining platelet count. For example, data from the CATCH registry suggested that < 10% of hospitalized patients receiving heparinoids who develop thrombocytopenia undergo appropriate screening for HIT.1
The risk of HIT varies, depending on the type of heparin exposure and patient population, but tends to be higher in women, post-surgical patients, and patients receiving unfractionated heparin (UFH) for a longer duration. In a prospective study of subcutaneous heparin (duration 10 ± 3 days) used for the prevention of deep vein thrombosis (DVT) in post-hip replacement patients, Warkentin et al30 demonstrated that 4.8% of patients developed HIT. The risk of HIT is lower in general medical and obstetrical populations.31,32 As an example, Creekmore et al33 found that HIT occurred in 43 of 8420 (0.51%) consecutive medical patients who received subcutaneous heparin for DVT prevention.
Although the mechanism is not completely understood, HIT develops when heparin binds to platelet factor-4 (PF4), forming a heparin: PF4 complex. Heparin-induced thrombocytopenia antibodies, usually of the IgG class, react with the heparin: PF4 complex to form a large macromolecule that activates platelets and monocytes,34 leading to the release of prothrombotic mediators.35
Low-molecular-weight heparins (LMWHs) have been associated with an 8- to 10-fold lower risk of HIT when compared with UFH,30,33 due to several factors.27 First, UFH has a larger number of negatively charged disaccharide chains compared with LMWH, optimizing the binding of these negative chains to positively charged PF4. Secondly, UFH has a higher number of sulfate groups than LMWH and ≥ 3 sulfate groups enhance the risk of HIT.36 Finally, because the HIT antigen only appears to form when particular stoichiometric concentrations of heparin and PF4 are present, levels of circulating PF4 may also influence the risk of developing HIT. Higher levels of PF4 are often found in cardiac and orthopedic surgery, and may be why HIT antibodies are found with such high prevalence in these patients.37
Although HIT may be less common now that LMWHs are used more extensively and, in some places, have replaced UFH, LMWH-induced HIT appears to be just as severe as UFH-induced HIT.32 Importantly, HIT can develop even with very minimal exposure to UFH (eg, heparin flushes through peripheral IVs or central catheters). When HIT develops, total hospitalization costs and lengths of stay increase significantly.33,38
Clinical Presentation of HIT
Classically, HIT is characterized by the development of thrombocytopenia 5 to 10 days after exposure to heparin. The thrombocytopenia is relative, not absolute, and defined as a decline in platelet count of ≥ 50% from the patient's baseline or postoperative peak platelet count. Importantly, postoperative patients often have a decrease of 30% from their preoperative platelet count, followed by a recovery during the first postoperative week. Heparin-induced thrombocytopenia is suggested in these surgical patients if they have a repeat postoperative decrease in their platelet count.30 About 10% to 15% of patients with HIT will have a normal platelet count (ie, > 150 000/μL), making absolute platelet count cutoffs an insensitive means of monitoring for onset. The mean platelet nadir in HIT is usually 50 000 to 60 000/μL, although rarely HIT patients may develop more severe thrombocytopenia (20 000–30 000/μL). Patients with HIT who have more severe thrombocytopenia appear to have a higher risk of amputation, thrombotic progression, and death.39
The most common form of HIT, occurring in about 60% of cases, is called “typical-onset HIT.” In this form, patients develop thrombocytopenia 5 to 10 days after heparin exposure. In the less common “rapid-onset HIT,” thrombocytopenia develops within 24 hours in patients recently exposed to heparin, usually within the past 30 days, who thus already have circulating HIT antibodies. Finally, a very uncommon form of HIT, called “delayed-onset HIT” is characterized by the development of thrombocytopenia ≥ 10 days after heparin exposure.32 Heparin-induced thrombocytopenia occurring in relation to fondaparinux and spontaneous HIT, and in the absence of any recognizable heparin exposure, have been described, but appear rare.40,41
Because thrombocytopenia is an easy clinical marker to use for the early diagnosis of HIT, the 2008 American College of Chest Physicians (ACCP) Evidence-Based Clinical Practice Guidelines recommend platelet count monitoring for patients receiving heparinoids, with the frequency of monitoring based on the patient's risk of HIT. For patients who have received heparin within the past 100 days, platelet counts should be obtained prior to and 24 hours after the readministration of heparin. Otherwise, for heparin-naïve patients, platelet monitoring, as recommended by the ACCP guidelines, should occur every other day in high-risk patients (therapeutic doses of heparin or prophylactic doses of UFH in postoperative patients) and every 2 to 3 days in moderate-risk patients (prophylactic doses of UFH in medically ill patients or prophylactic doses of LMWH in postoperative patients). Routine platelet count monitoring in obstetric or medical patients receiving LMWH is usually unnecessary.42
The risk of thrombosis is high as HIT develops and although venous thrombosis, including pulmonary embolism and DVT, occurs 4 times more often than arterial thrombosis (such as limb ischemia, myocardial infarction, and stroke), arterial events cause more morbidity and mortality.43,44 In up to 15% of patients, new thromboses can precede the development of thrombocytopenia.42
Diagnosis of HIT
Heparin-induced thrombocytopenia is a “clinical-pathologic” diagnosis—one in which combining a pre-test clinical probability assessment with laboratory testing is important to accurately exclude or rule-in the diagnosis. Reliance on laboratory testing alone, without consideration of the pre-test probability (PTP), may lead to the overdiagnosis and treatment of HIT.45 The prevalence of circulating and measurable HIT antibodies varies based on the patient population and heparin exposure. For example, up to 20% of orthopedic surgery and 70% of cardiopulmonary bypass patients may have circulating HIT antibodies.46 However, in the majority of these patients, the antibodies are not pathogenic and do not activate platelets and trigger the thrombotic cascade. Of these patients with measurable heparin antibodies, only a small subset ever develop pathogenic HIT antibodies that can induce the clinical syndrome of HIT.47
There are 2 forms of laboratory-based tests for HIT: antibody assays, such as the enzyme immunoassay, and platelet activation tests, such as the serotonin-release assay (SRA). Enzyme immunoassays have a high sensitivity (up to 99%) and, if negative, can be used to exclude HIT, but do not distinguish between pathogenic and nonpathogenic antibodies. Therefore, these assays are excellent for ruling out HIT (ie, have a high negative predictive value) but, because they are not specific, are less helpful for confirming the diagnosis (ie, have a moderate positive predictive value). Because the enzyme immunoassays employ a chromogenic reaction, a measurable optical density (OD) can be obtained. Most antibody assays provide a manufacturer's cutoff for an OD of 0.4 and, depending on the hospital and/or laboratory, HIT antibody test results may be given a qualitative (positive/negative) interpretation or a quantitative value (OD units). If an actual value is provided, HIT is more likely if the OD is > 1.0 and less likely with weak positive results (OD, 0.4–1.0).48,49
The SRA, the gold standard, exposes washed donor platelets labeled with 14C-serotonin to plasma or serum of suspected HIT patients, in the presence of either a low or high heparin concentration. If pathogenic HIT antibodies are present in the patient's serum/plasma, the donor platelets will be activated with low heparin concentrations, leading to 14C-serotonin release and subsequent detection. This reaction is suppressed with high heparin concentrations, providing a “2-point approach” and giving the test its high specificity.32 A positive SRA is consistent with a diagnosis of HIT but, unfortunately, this test is not widely available and has a longer turnaround time, limiting its routine practicality. These tests may be used in combination with each other and a clinical scoring system, the 4T's, for clinical decision making. If both tests are negative, HIT may be excluded, and when both tests are positive, HIT is usually diagnosed. When the tests are discordant, because the SRA is considered the gold standard, it is generally considered a more reliable method for diagnosing HIT.
A validated clinical scoring system has been developed and published and is clinically helpful when evaluating patients with suspected HIT. Called the “4T's,” this clinical scoring system allows for the determination of a PTP that, in combination with HIT laboratory testing, allows the hospitalist physician to more accurately confirm or exclude a diagnosis of HIT (Table 3). The negative predictive value of a low PTP alone (ie, a score ≤ 3), without HIT antibody testing, has been and suggested to be approximately 100%, although it has not been well studied in all patients.50 Because of this high negative predictive value, the routine ordering of HIT antibody tests in patients with low PTPs may not be necessary.50–52 In patients with a moderate PTP (score of 4–5 points), it is reasonable to stop all heparin products and order the HIT antibody test; initiation of a direct thrombin inhibitor (DTI) may be warranted depending on the clinical suspicion. Finally, in patients with a high PTP (score of 6–8 points), all heparin products should be discontinued, the HIT antibody test ordered, and administration of a DTI, such as argatroban or lepirudin, should be considered if bleeding risk is acceptably low.42 Finally, because of the limitations of the HIT antibody tests, current guidelines recommend against routine HIT antibody testing if patients do not have thrombocytopenia, thrombosis, heparin-induced skin lesions, or other evidence of HIT.42
Table 3. Establishing the Pre-Test Probability for Suspected Heparin-induced Thrombocytopenia: The 4T's.
| The 4T's | 2 Points | 1 Point | 0 Points |
|---|---|---|---|
| Thrombocytopenia | > 50% fall or nadir of 20–100 × 109 cells/L | 30%–50% fall or nadir 10–19 × 109 cells/L | < 30% fall or nadir < 10 × 109 cells/L |
| Timing of thrombocytopenia (relative to heparin) | 5–10 days or < 1 day of heparin reexposure within 30 days | Beyond day 10 or unclear | < 5 days no reexposure |
| Thrombosis | Proven (skin necrosis, thrombosis) | Recurrent or suspected | None |
| eTiology of thrombocytopenia | No other cause evident | Possible | Definite |
Scoring: 0–3, low probability; 4—5, moderate probability; 6–8, high probability.
Adapted from Curr Hematai Rep.56
Treatment of HIT
The initial management of HIT, when strongly suspected or confirmed, involves immediate discontinuation of all heparinoids (including UFH and LMWH) and, if there are no contraindications, initiation of a DTI. Because the risk of thrombosis in HIT patients managed by heparin discontinuation alone is approximately 50%, cessation of heparinoids alone is inadequate.53 There are several DTIs available for the treatment of HIT, including 3 FDA-approved drugs, argatroban, bivalirudin, and lepirudin, all of which are parenterally administered. A comprehensive discussion on the treatment of HIT is beyond the scope of this article and the reader is referred to the 2008 ACCP guidelines for a more thorough discussion.42
Argatroban, which is currently approved by the FDA for the treatment of HIT and for use during percutaneous coronary intervention, has minimal renal clearance and a rapid onset of action. The initial FDA-approved infusion rate of argatroban is 2 μg/kg/min (no bolus) with dose adjustments to maintain an activated partial thromboplastin time (aPTT) of 1.5 to 3 times the normal rate. Smaller starting doses should be used in critically ill patients or those with hepatic insufficiency.32
Lepirudin is also approved for the treatment of acute HIT but differs from argatroban in several ways, including predominant renal elimination (requiring dose reduction to ≤ 0.05–0.10 mg/kg/h) and the risk of fatal anaphylaxis due to preexisting hirudin antibodies in patients with previous lepirudin exposure.54 Patients should be informed of this risk and the bolus should be omitted to minimize the risk of anaphylaxis.48 Patients on lepirudin should be monitored with aPTT every 4 hours until a steady state within therapeutic range is achieved.
Bivalirudin in HIT is less well studied and not FDA-approved for the treatment of HIT in patients not undergoing percutaneous coronary intervention. Bivalirudin dosing is not well established but a reasonable dosing regimen is 0.15 mg/kg/h with dose adjustments to maintain an aPTT 1.5 to 2.5 times baseline. Patients with either renal and/or hepatic dysfunction require an approximately 50% dose reduction.32
Once a DTI has been started and the platelet count is recovering to ≥ 150 000/μL (suggesting that the thrombotic processes are controlled), warfarin may be started. Starting warfarin prior to platelet recovery may precipitate limb gangrene (due to transient protein C reductions) and/or skin necrosis. Warfarin should be overlapped with a DTI for 4 to 5 days and until 2 therapeutic international normalized ratios (INRs) have been achieved, although the duration of overlap has not been well studied. Because argatroban prolongs the INR, care should be taken not to discontinue argatroban prematurely; published algorithms exist to guide hospitalists on this transition.42
Platelet transfusions should be avoided in HIT and although small, recently published case reports suggest that they may be safe if absolutely indicated, more convincing data are needed.55 Patients with HIT who must undergo cardiopulmonary bypass surgery represent a challenging population. Ideally, the surgery or intervention should be delayed until acute HIT has resolved. Current guidelines recommend 1 of the following, in descending order of preference: 1) delay surgery until platelet recovery and the antibody is undetectable; 2) use of bivalirudin during cardiopulmonary bypass; 3) use of lepirudin during off-pump cardiac surgery; or 4) use of UFH plus epoprostenol, UFH plus tirofiban, or danaparoid. If UFH is used, exposure to UFH should be limited to the intraoperative period only. Pre- and postoperatively, nonheparin anticoagulants should be administered.42
Conclusion
In hospitalized patients, the development of acute thrombocytopenia is not uncommon and should prompt the consideration of medications as a possible etiology, especially when an obvious explanation is not evident. Drug-induced thrombocytopenia can cause significant morbidity, including life-threatening bleeding, but must be distinguished from HIT, associated with limb- and life-threatening thrombosis. When HIT is suspected, a clinical PTP can be estimated using the 4T's scoring system and, in cases of moderate or high probability, heparin should be immediately discontinued, appropriate diagnostic testing initiated, and alternative nonheparin anticoagulants started.
Acknowledgments
We are indebted to Ms. Kim Mahoney for her design assistance and Ms. Sharla Watts for her editorial assistance.
Footnotes
Conflict of Interest Statement: Matthew T. Rondina, MD discloses conflicts of interest with sanofi-aventis. Amanda Walker, PharmD and Robert C. Pendleton, MD disclose no conflicts of interest.
References
- 1.Oliveira GB, Crespo EM, Becker RC, et al. Complications After Thrombocytopenia Caused by Heparin (CATCH) Registry Investigators. Incidence and prognostic significance of thrombocytopenia in patients treated with prolonged heparin therapy. Arch Intern Med. 2008;168(1):94–102. doi: 10.1001/archinternmed.2007.65. [DOI] [PubMed] [Google Scholar]
- 2.Wang TY, Ou FS, Roe MT, et al. Incidence and prognostic significance of thrombocytopenia developed during acute coronary syndrome in contemporary clinical practice. Circulation. 2009;119(18):2454–2462. doi: 10.1161/CIRCULATIONAHA.108.827162. [DOI] [PubMed] [Google Scholar]
- 3.Hambleton J, Shuman MA. Hemorrhagic and thrombotic disorders in hospital medicine. In: Wachter RM, Goldman L, Hollander H, editors. Hospital Medicine. 2nd. Philadelphia, PA: Lippincott, Williams & Wilkins; 2005. pp. 592–600. [Google Scholar]
- 4.George JN, Raskob GE, Shah SR, et al. Drug-induced thrombocytopenia: a systematic review of published case reports. Ann Intern Med. 1998;129(11):886–890. doi: 10.7326/0003-4819-129-11_part_1-199812010-00009. [DOI] [PubMed] [Google Scholar]
- 5.Laber DA, Martin ME. Etiology of thrombocytopenia in all patients treated with heparin products. Eur J Haematol. 2005;75(2):101–105. doi: 10.1111/j.1600-0609.2005.00503.x. [DOI] [PubMed] [Google Scholar]
- 6.George JN, Aster RH. Drug-induced thrombocytopenia: pathogenesis, evaluation, and management. Hematology Am Soc Hematol Educ Program. 2009:153–158. doi: 10.1182/asheducation-2009.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Bemt PM, Meyboom RH, Egberts AC. Drug-induced immune thrombocytopenia. Drug Saf. 2004;27(15):1243–1252. doi: 10.2165/00002018-200427150-00007. [DOI] [PubMed] [Google Scholar]
- 8.Kenney B, Stack G. Drug-induced thrombocytopenia. Arch Pathol Lab Med. 2009;133(2):309–314. doi: 10.5858/133.2.309. [DOI] [PubMed] [Google Scholar]
- 9.Visentin GP, Liu CY. Drug-induced thrombocytopenia. Hematol Oncol Clin North Am. 2007;21(4):685–696. vi. doi: 10.1016/j.hoc.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aster RH. Immune thrombocytopenia caused by glycoprotein IIb/IIIa inhibitors. Chest. 2005;127(2 suppl):53S–59S. doi: 10.1378/chest.127.2_suppl.53S. [DOI] [PubMed] [Google Scholar]
- 11.Bougie DW, Wilker PR, Wuitschick ED, et al. Acute thrombocytopenia after treatment with tirofiban or eptifibatide is associated with antibodies specific for ligand-occupied GPIIb/IIIa. Blood. 2002;100(6):2071–2076. [PubMed] [Google Scholar]
- 12.Jubelirer SJ, Koenig BA, Bates MC. Acute profound thrombocytopenia following C7E3 Fab (Abciximab) therapy: case reports, review of the literature and implications for therapy. Am J Hematol. 1999;61(3):205–208. doi: 10.1002/(sici)1096-8652(199907)61:3<205::aid-ajh8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Wazny LD, Ariano RE. Evaluation and management of drug-induced thrombocytopenia in the acutely ill patient. Pharmacotherapy. 2000;20(3):292–307. doi: 10.1592/phco.20.4.292.34883. [DOI] [PubMed] [Google Scholar]
- 14.Dery JP, Braden GA, Lincoff AM, et al. ReoPro Readministration Registry Investigators. Final results of the ReoPro readministration registry. Am J Cardiol. 2004;93(8):979–984. doi: 10.1016/j.amjcard.2003.12.051. [DOI] [PubMed] [Google Scholar]
- 15.Von Drygalski A, Curtis BR, Bougie DW, et al. Vancomycin-induced immune thrombocytopenia. N Engl J Med. 2007;356(9):904–910. doi: 10.1056/NEJMoa065066. [DOI] [PubMed] [Google Scholar]
- 16.Gerson SL, Kaplan SL, Bruss JB, et al. Hematologic effects of linezolid: summary of clinical experience. Antimicrob Agents Chemother. 2002;46(8):2723–2726. doi: 10.1128/AAC.46.8.2723-2726.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu VC, Wang YT, Wang CY, et al. High frequency of linezolid-associated thrombocytopenia and anemia among patients with end-stage renal disease. Clin Infect Dis. 2006;42(1):66–72. doi: 10.1086/498509. [DOI] [PubMed] [Google Scholar]
- 18.Niwa T, Suzuki A, Sakakibara S, et al. Retrospective cohort chart review study of factors associated with the development of thrombocytopenia in adult Japanese patients who received intravenous linezolid therapy. Clin Ther. 2009;31(10):2126–2133. doi: 10.1016/j.clinthera.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Aster RH, Curtis BR, McFarland JG, Bougie DW. Drug-induced immune thrombocytopenia: pathogenesis, diagnosis, and management. J Thromb Haemost. 2009;7(6):911–918. doi: 10.1111/j.1538-7836.2009.03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy JC, Shuman MA, Aster RH. Quinine/quinidine-induced thrombocytopenia: a great imitator. Arch Intern Med. 2004;164(2):218–220. doi: 10.1001/archinte.164.2.218. [DOI] [PubMed] [Google Scholar]
- 21.Gottschall JL, Elliot W, Lianos E, McFarland JG, Wolfmeyer K, Aster RH. Quinine-induced immune thrombocytopenia associated with hemolytic uremic syndrome: a new clinical entity. Blood. 1991;77(2):306–310. [PubMed] [Google Scholar]
- 22.Freiman JP. Fatal quinine-induced thrombocytopenia. Ann Intern Med. 1990;112(4):308–309. doi: 10.7326/0003-4819-112-4-308. [DOI] [PubMed] [Google Scholar]
- 23.Brasić JR. Quinine-induced thrombocytopenia in a 64-year-old man who consumed tonic water to relieve nocturnal leg cramps. Mayo Clin Proc. 2001;76(8):863–864. doi: 10.1016/s0025-6196(11)63235-7. [DOI] [PubMed] [Google Scholar]
- 24.Pourrat O. Treatment of drug-related diseases by plasma exchanges. Ann Med Interne (Paris) 1994;145(5):357–360. [PubMed] [Google Scholar]
- 25.Ray JB, Brereton WF, Nullet FR. Intravenous immune globulin for the treatment of presumed quinidine-induced thrombocytopenia. DICP. 1990;24(7–8):693–695. doi: 10.1177/106002809002400706. [DOI] [PubMed] [Google Scholar]
- 26.Stasi R, Evangelista ML, Amadori S. Novel thrombopoietic agents: a review of their use in idiopathic thrombocytopenic purpura. Drugs. 2008;68(7):901–912. doi: 10.2165/00003495-200868070-00002. [DOI] [PubMed] [Google Scholar]
- 27.Jang IK, Hursting MJ. When heparins promote thrombosis: review of heparin-induced thrombocytopenia. Circulation. 2005;111(20):2671–2683. doi: 10.1161/CIRCULATIONAHA.104.518563. [DOI] [PubMed] [Google Scholar]
- 28.Burgess JK, Chong BH. The platelet proaggregating and potentiating effects of unfractionated heparin, low molecular weight heparin and heparinoid in intensive care patients and healthy controls. Eur J Haematol. 1997;58(4):279–285. doi: 10.1111/j.1600-0609.1997.tb01667.x. [DOI] [PubMed] [Google Scholar]
- 29.Chong BH, Castaldi PA. Platelet proaggregating effect of heparin: possible mechanism for non-immune heparin-associated thrombocytopenia. Aust N Z J Med. 1986;16(5):715–716. doi: 10.1111/j.1445-5994.1986.tb00023.x. [DOI] [PubMed] [Google Scholar]
- 30.Warkentin TE, Roberts RS, Hirsh J, Kelton JG. An improved definition of immune heparin-induced thrombocytopenia in postoperative orthopedic patients. Arch Intern Med. 2003;163(20):2518–2524. doi: 10.1001/archinte.163.20.2518. [DOI] [PubMed] [Google Scholar]
- 31.Dager WE, White RH. Pharmacotherapy of heparin-induced thrombocytopenia. Expert Opin Pharmacother. 2003;4(6):919–940. doi: 10.1517/14656566.4.6.919. [DOI] [PubMed] [Google Scholar]
- 32.Shantsila E, Lip GY, Chong BH. Heparin-induced thrombocytopenia. A contemporary clinical approach to diagnosis and management. Chest. 2009;135(6):1651–1664. doi: 10.1378/chest.08-2830. [DOI] [PubMed] [Google Scholar]
- 33.Creekmore FM, Oderda GM, Pendleton RC, Brixner DI. Incidence and economic implications of heparin-induced thrombocytopenia in medical patients receiving prophylaxis for venous thromboembolism. Pharmacotherapy. 2006;26(10):1438–1445. doi: 10.1592/phco.26.10.1438. [DOI] [PubMed] [Google Scholar]
- 34.Rauova L, Zhai L, Kowalska MA, Arepally GM, Cines DB, Poncz M. Role of platelet surface PF4 antigenic complexes in heparin-induced thrombocytopenia pathogenesis: diagnostic and therapeutic implications. Blood. 2006;107(6):2346–2353. doi: 10.1182/blood-2005-08-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chong BH, Grace CS, Rozenberg MC. Heparin-induced thrombocytopenia: effect of heparin platelet antibody on platelets. Br J Haematol. 1981;49(4):531–540. doi: 10.1111/j.1365-2141.1981.tb07261.x. [DOI] [PubMed] [Google Scholar]
- 36.Walenga JM, Jeske WP, Prechel MM, Bacher P, Bakhos M. Decreased prevalence of heparin-induced thrombocytopenia with low-molecular-weight heparin and related drugs. Semin Thromb Hemost. 2004;30(suppl l):69–80. doi: 10.1055/s-2004-823005. [DOI] [PubMed] [Google Scholar]
- 37.Warkentin TE, Greinacher A. Heparin-induced thrombocytopenia and cardiac surgery. Ann Thorac Surg. 2003;76(2):638–648. doi: 10.1016/s0003-4975(03)00756-2. [DOI] [PubMed] [Google Scholar]
- 38.Smythe MA, Koerber JM, Fitzgerald M, Mattson JC. The financial impact of heparin-induced thrombocytopenia. Chest. 2008;134(3):568–573. doi: 10.1378/chest.08-0120. [DOI] [PubMed] [Google Scholar]
- 39.Kelton JG, Hursting MJ, Heddle N, Lewis BE. Predictors of clinical outcome in patients with heparin-induced thrombocytopenia treated with direct thrombin inhibition. Blood Coagul Fibrinolysis. 2008;19(6):471–475. doi: 10.1097/MBC.0b013e3282a167cc. [DOI] [PubMed] [Google Scholar]
- 40.Warkentin TE, Makris M, Jay RM, Kelton JG. A spontaneous prothrombotic disorder resembling heparin-induced thrombocytopenia. Am J Med. 2008;121(7):632–636. doi: 10.1016/j.amjmed.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 41.Warkentin TE, Maurer BT, Aster RH. Heparin-induced thrombocytopenia associated with fondaparinux. N Engl J Med. 2007;356(25):2653–2655. doi: 10.1056/NEJMc070346. [DOI] [PubMed] [Google Scholar]
- 42.Warkentin TE, Greinacher A, Koster A, Lincoff AM. American College of Chest Physicians. Treatment and prevention of heparin-induced thrombocytopenia: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (8th) 2008;133(6 suppl):340S–380S. doi: 10.1378/chest.08-0677. [DOI] [PubMed] [Google Scholar]
- 43.King DJ, Kelton JG. Heparin-associated thrombocytopenia. Ann Intern Med. 1984;100(4):535–540. doi: 10.7326/0003-4819-100-4-535. [DOI] [PubMed] [Google Scholar]
- 44.Warkentin TE. Clinical presentation of heparin-induced thrombocytopenia. Semin Hematol. 1998;35(4 suppl 5):9–16. [PubMed] [Google Scholar]
- 45.Pouplard C, Gueret P, Fouassier M, et al. Prospective evaluation of the ‘4Ts’ score and particle gel immunoassay specific to heparin/PF4 for the diagnosis of heparin-induced thrombocytopenia. J Thromb Haemost. 2007;5(7):1373–1379. doi: 10.1111/j.1538-7836.2007.02524.x. [DOI] [PubMed] [Google Scholar]
- 46.Amiral J, Peynaud-Debayle E, Wolf M, Bridey F, Vissac AM, Meyer D. Generation of antibodies to heparin-PF4 complexes without thrombocytopenia in patients treated with unfractionated or low-molecular-weight heparin. Am J Hematol. 1996;52(2):90–95. doi: 10.1002/(SICI)1096-8652(199606)52:2<90::AID-AJH4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 47.Warkentin TE. Heparin-induced thrombocytopenia. Pathogenesis, frequency, avoidance and management. Drug Saf. 1997;17(5):325–341. doi: 10.2165/00002018-199717050-00005. [DOI] [PubMed] [Google Scholar]
- 48.Greinacher A. Heparin-induced thrombocytopenia. J Thromb Haemost. 2009;7(suppl 1):9–12. doi: 10.1111/j.1538-7836.2009.03385.x. [DOI] [PubMed] [Google Scholar]
- 49.Whitlatch NL, Perry SL, Ortel TL. Anti-heparin/platelet factor 4 antibody optical density values and the confirmatory procedure in the diagnosis of heparin-induced thrombocytopenia. Thromb Haemost. 2008;100(4):678–684. doi: 10.1160/th08-02-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lo GK, Juhl D, Warkentin TE, Sigouin CS, Eichler P, Greinacher A. Evaluation of pretest clinical score (4 T's) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4(4):759–765. doi: 10.1111/j.1538-7836.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 51.Denys B, Stove V, Philippé J, Devreese K. A clinical-laboratory approach contributing to a rapid and reliable diagnosis of heparin-induced thrombocytopenia. Thromb Res. 2008;123(1):137–145. doi: 10.1016/j.thromres.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 52.Gruel Y, Régina S, Pouplard C. Usefulness of pretest clinical score (4Ts) combined with immunoassay for the diagnosis of heparin-induced thrombocytopenia. Curr Opin Pulm Med. 2008;14(5):397–402. doi: 10.1097/MCP.0b013e3283056507. [DOI] [PubMed] [Google Scholar]
- 53.Warkentin TE, Kelton JG. A 14-year study of heparin-induced thrombocytopenia. Am J Med. 1996;101(5):502–507. doi: 10.1016/s0002-9343(96)00258-6. [DOI] [PubMed] [Google Scholar]
- 54.Greinacher A, Lubenow N, Eichler P. Anaphylactic and anaphylactoid reactions associated with lepirudin in patients with heparin-induced thrombocytopenia. Circulation. 2003;108(17):2062–2065. doi: 10.1161/01.CIR.0000096056.37269.14. [DOI] [PubMed] [Google Scholar]
- 55.Hopkins CK, Goldfinger D. Platelet transfusions in heparin-induced thrombocytopenia: a report of four cases and review of the literature. Transfusion. 2008;48(10):2128–2l32. doi: 10.1111/j.1537-2995.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 56.Warkentin TE, Heddle NM. Laboratory diagnosis of immune heparin-induced thrombocytopenia. Curr Hematol Rep. 2003;2(2):148–157. [PubMed] [Google Scholar]