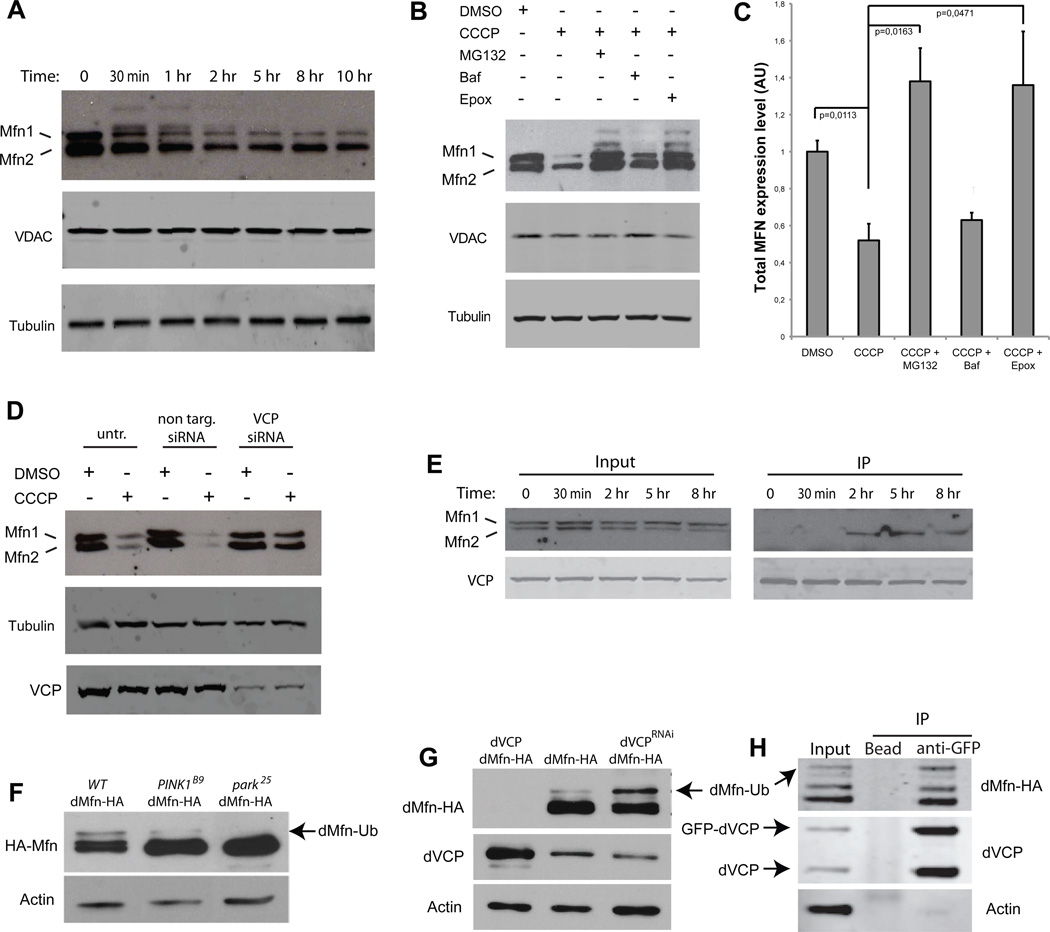
Figure 6. Mitofusin degradation by the proteasome is dependent on VCP.
A. Western blots in YFP-Parkin stable HeLa cells against MFN1 and 2, VDAC and tubulin at different time points after CCCP treatment. Ubiquitinated forms of MFNs 1/2 can be observed migrating more slowly. B. Western blots in YFP-Parkin stable HeLa cells against MFN1/2, VDAC, and tubulin. Cells were treated for 12 h with CCCP and either proteasome inhibitors (MG132 or epoxomicin) or the autophagy inhibitor bafilomycin. Ubiquitinated forms of MFN1/2 can be again observed migrating more slowly, particularly with proteasome inhibition. C. Quantification of total MFN expression levels normalized against tubulin in YFP-Parkin stable HeLa cells treated for 12 h with CCCP and either proteasome inhibitors (MG132 or epoxomicin), or the autophagy inhibitor bafilomycin. Error bars indicate standard deviation from triplicates. D. Knockdown of VCP stabilizes MFNs 1 and 2. Western blots in YFP-Parkin stable HeLa cells against MFN1/2, VCP and tubulin. Cells were transfected with non-targeting or VCP-targeting siRNA and treated for 12 h with DMSO or CCCP. E. FLAG IP in HeLa cells cotransfected with YFP-Parkin and VCP-FLAG and treated with CCCP for the indicated times. Immunoprecipitation samples were immunoblotted against MFN1/2 and VCP. Following mitochondrial depolarization VCP interacts with MFN2. F. Total dMfn-HA accumulates in PINK1B9 (lane 2) and Park25 (lane 3) null mutants. Notably, ubiquitinated dMfn is decreased in PINK1B9 null mutants (lane 2) and absent in Park25 null mutants (lane 3). G. Overexpression of dVCP in the compound eye destabilizes dMfn-HA (lane 1), whereas knockdown of endogenous dVCP in the compound eye leads to accumulation of ubiquitinated dMfn-HA (lane 3). H. Immunoprecipitation of HA-dMfn and endogenous (protein-trap) GFP-dVCP from Drosophila brain extract.