Figure 2. Potential mechanism for inhibiting CB function in transformed cells.
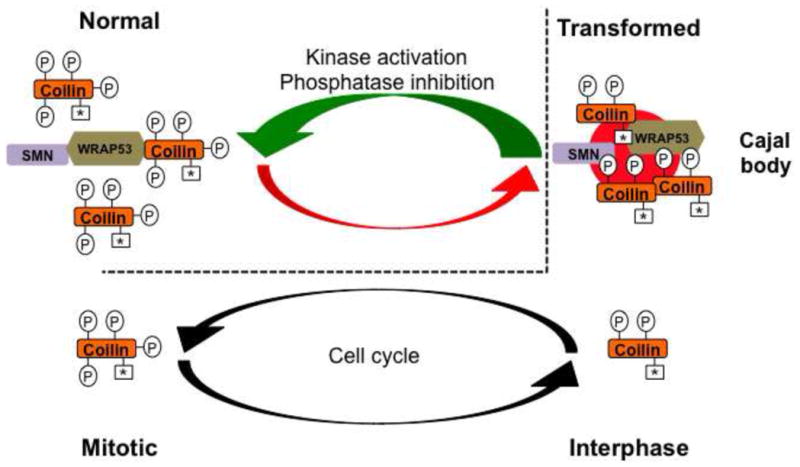
Coilin is relatively hyperphosphorylated in most normal cells (denoted by four Ps) compared to that in transformed cells (two Ps). Because hyperphosphorylated coilin has reduced self-association, CBs do not form in most normal cells. However, the extent of coilin phosphorylation is reduced in transformed cells, which correlates with increased coilin self-association and CB formation. A parallel can be drawn between the amount of phosphorylation of coilin in normal cells to that of mitotic coilin in transformed cells (dashed line). In transformed cells, interphase coilin is phosphorylated but can still self-associate and form CBs (two Ps). When transformed cells enter mitosis, however, CBs disassemble and this disassembly correlates with increased coilin phosphorylation (four Ps). We speculate that this same level or extent of coilin phosphorylation in mitotic transformed cells is also present during interphase of normal cells (note the four Ps in normal cells), and this accounts for the lack of CBs in most normal cell types. Strategies that could activate kinases or inhibit phosphatases that act on coilin would be detrimental to transformed cells (green arrow) because the lack of CBs would decrease the available snRNP and telomerase resources, and thus make these cells more “normal” in regards to nuclear organization. Symmetrical dimethylarginine modifications on coilin are indicated by an asterisk (*).
