Abstract
Study’s purpose: Plasma levels of soluble receptor for advanced glycation endproducts (sRAGE) and S100A12 are increased in young children after cardiac surgery and correlate with the time spent on cardiopulmonary bypass (CPB). This study was performed to investigate whether plasma levels of sRAGE and S100A12 are affected by the use of CPB. Levels of S100A12 and sRAGE, along with of interleukin-6, tumor necrosis factor-α, myeloperoxidase, and C-reactive protein were measured in 25 adults undergoing non-urgent coronary artery bypass grafting with and without the use of CPB. Significant finding: Plasma levels of S100A12, sRAGE, IL-6, TNF-α and MPO 4h after cardiac surgery were elevated compared to baseline; this increase was equally observed in patients undergoing traditional coronary artery bypass grafting on cardiopulmonary bypass (n = 16), and in patients undergoing robot-assisted coronary artery bypass grafting off pump (OPCAB, n = 9). Patients with prolonged hospitalization of 7 days or longer had significantly higher S100A12 and sRAGE 4 hours post surgery compared to patients hospitalized ≤ 6 days. Conclusion: Increased sRAGE and S100A12 after cardiac surgery is associated with prolonged length of hospitalization in patients after coronary artery bypass grafting; however, we did not observe an intrinsic effect of cardiopulmonary bypass on S100A12 or sRAGE plasma levels in our small pilot study. Further studies are required to confirm the value of sRAGE and S100A12 in predicting postoperative complications after cardiac surgery in a larger study.
Keywords: Coronary artery bypass graft surgery, S100/calgranulin, sRAGE, biomarker
Introduction
The systemic inflammatory response in patients undergoing cardiac surgery contributes substantially to postoperative organ dysfunction, morbidity and mortality. The degree of cytokine release during and after cardiac surgery varies considerable among different studies and this is likely influenced by many factors and processes such as tissue damage, reperfusion injury, peri-operatively administered drugs and hemodilution, as well as inter-individual cytokine responses to injury [1-3]. Particularly, exposure of blood to non-endothelial surfaces in cardiopulmonary bypass (CPB) is debated as a major contributor to the systemic inflammatory response in patients undergoing coronary artery bypass grafting (reviewed in [4]).
S100A12 is a member of the S100 family of calcium binding proteins and highly expressed endogenously in neutrophilic granulocytes and lesser in other cells. Accordingly, elevated serum concentration of S100A12 is an emerging biomarker of various chronic and acute inflammatory diseases, including acute lung injury [5-7]. Due to the ability to activate RAGE, a multi-ligand cell surface receptor abundantly expressed in alveolar lung cells and other cells, there is growing evidence that S100A12 may modulates inflammation [8,9]. Liu et al. recently reported on elevated plasma levels of S100A12 and sRAGE immediately after CPB in 58 children age < 3 years undergoing non-urgent surgery for congenital heart disease [10]. In this study, there was a positive correlation of S100A12 and sRAGE plasma levels with incidence of acute lung injury and with prolonged length of hospitalization. Additionally, Liu et al. reported a strong positive correlation between the time spent on CPB and the increase in these biomarkers post-surgery, raising the hypothesis that altered hemodynamics or contact with non-endothelial surfaces adherent to CPB could possibly affect plasma concentration of S100A12 and sRAGE. In the here presented study we examine the impact of CPB on plasma concentrations of S100A12 and sRAGE in a small non-randomized single center observational study of consecutive patients undergoing elective coronary artery bypass grafting.
Materials and methods
Blood samples were prospectively collected on 25 consecutive adult patients undergoing non-urgent coronary artery bypass grafting surgery at The University of Chicago Medical Center. We excluded patients with myocardial infarction within 6 weeks prior surgery, severe left ventricular dysfunction (ejection fraction < 30%), chronic kidney disease > stage III, emergency surgery after angiography or percutaneous coronary intervention. Of those 25 patients, 9 subjects had off pump coronary artery bypass (OPCAP) using the da Vinci robot, and 16 patients had conventional coronary artery bypass grafting (CABG with CPB). Patients were not randomized and the surgeon in consultation with the patient made the decision for OPCAP versus CABG with CPD. Three experienced cardiac surgeons operated on all 25 patients with no overlap between OPCAP and CABG with CPD. All patients underwent routine preoperative and postoperative care directed by the multidisciplinary surgical and medical team. The operative risk before surgery was prospectively calculated using the additive and logistic European System for Cardiac Operative Risk Evaluation (Euro-SCORE [11]. An echo board certified reader measured the left ventricular ejection fraction (LV-EF) on transthoracic echocardiograms prior surgery. All other demographic data were collected retrospectively from the electronic medical records. Diabetes was defined by the use of oral medication or insulin prior surgery or upon discharge, hypertension was defined as systolic blood pressure > 140 before surgery or the use of antihypertensive medication, COPD was defined by documented moderately or severely abnormal pulmonary function tests or the use of inhaler. Hospital length of stay (LOS) was defined as days spent in the hospital after coronary artery bypass grafting surgery. Discharge from the hospital occurred daily and was determined by the surgical and medical team.
Blood samples were collected immediately prior (0 h, in the operating room) and 4 h after completed cardiac surgery using a vacuum tube containing EDTA. After centrifugation at 3000 rpm for 15 min at 4°C, plasma aliquots were stored at -80°C until use. S100A12, sRAGE, IL-6, TNF-α, MPO, and CRP were selected as candidate biomarker and measured using commercially available ELISA kits (R&D systems), or as previously published for S100A12 [12]. Human plasma was obtained with informed consent and approved by the University of Chicago Institutional Review Board.
Descriptive statistics (percentage, mean, and standard deviation), crude comparison based on chi-square statistics and plots were used to describe the patient cohort. All continuous data are reported as mean ± SD. The independent-sample t-test and one-way analysis of variance were used for mean comparison between the 2 groups. We considered differences significant when p values were less than or equal to 0.05.
Results
Baseline characteristics were similar between patients assigned to conventional coronary artery bypass grafting surgery and those assigned to off pump coronary artery bypass grafting surgery (Table 1), except a higher frequency of COPD in the OPCAB group with 2/9 patients having COPD compared to 1/16 in the conventional group (Table 1). No cross over occurred between the two groups.
Table 1.
Preoperative characteristics of patients undergoing coronary artery bypass grafting with cardiopulmonary bypass (CABG with CPB) or off pump coronary artery bypass (OPCAB)
| All patients (n = 25) | CABG with CPB (n = 16) | OPCAB (n = 9) | P-value | |
|---|---|---|---|---|
| Mean (SD) age (years) | 60.9 (8.3) | 58 (7.8) | 60 (8.1) | 0.27 |
| Male | 17 (68) | 12 (75) | 5 (55) | 0.36 |
| Current smoker | 10 (40) | 6 (37) | 4 (44) | 0.2 |
| LV EF > 50% | 14 (56) | 8 (50) | 6 (67) | 0.3 |
| LV EF 30%-49% | 11 (44) | 8 (50) | 3 (33) | 0.6 |
| Diabetes | 9 (36) | 6 (37) | 3 (33) | 0.6 |
| Hypertension | 20 (80) | 14 (87) | 6 (66) | 0.4 |
| Statin use | 19 (76) | 11 (68) | 8 (88) | 0.2 |
| COPD | 3 (12) | 1 (6) | 2 (22) | 0.04 |
| Euro-Score | 8.7 | 8.4 | 8.8 | 0.34 |
| Mean (SD) Operation time (min) | 302 (56) | 312 (45) | 290 (78) | 0.43 |
| In-hospital mortality | 0 | 0 | 0 | |
| Mean (SD) Hospital LOS (days) | 7.2 (5.3) | 7.4 (6.7) | 6.2 (4.1) | 0.35 |
Values are numbers (percentages) unless stated otherwise. (LOS, length of stay).
Similar to the findings of Liu et al. [10], we found a strong increase in plasma sRAGE (1976 pg/ml ± 671 vs. 765 pg/ml ± 246; p = 0.00018) and S100A12 (3458 ng/ml ± 420 vs. 85 ng/ml ± 65; p = 0.00062) taken 4 h after completed cardiac surgery compared to those taken immediately prior to cardiac surgery (Table 2). Furthermore, IL-6 (431 pg/ml ± 210 vs. 14 pg/ml ± 21; p = 0.00038), MPO (23.5 ng/ml ± 11 vs. 9.4 ng/ml ± 4.8, p < 0.00001), and TNF-α (9.6 pg/ml ± 4.1 vs. 5.2 pg/ml ± 4.1; p = 0.04) were also significantly increased postoperatively, but not CRP (0.9 µg/ml ± 1.3 vs. 1.4 µg/ml, ± 3.1; p = 0.226).
Table 2.
Plasma biomarker concentration in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass (CABG with CPB) or off pump coronary artery bypass (OPCAB)
| All patients (n = 25) | CABG with CPB (n = 16) | OPCAB (n = 9) | P-value | |
|---|---|---|---|---|
| sRAGE, 0 h, pg/ml | 765 (246) | 659 (221) | 771 (233) | 0.273 |
| sRAGE, 4 h, pg/ml | 1976 (671)* | 2145 (632)* | 1732 (634)* | 0.432 |
| S100A12, 0 h, ng/ml | 85 (65) | 88 (49) | 68 (62) | 0.385 |
| S100A12, 4 h, ng/ml | 3458 (420)* | 2956 (379)* | 3638 (420)* | 0.276 |
| Il-6, 0 h, pg/ml | 14 (21) | 13 (19) | 14 (22) | 0.456 |
| IL-6, 4 h, pg/ml | 431 (210)* | 386 (194)* | 482 (170)* | 0.441 |
| MPO, 0 h, ng/ml | 9.4 (4.8) | 8.3 (3.8) | 9.6 (4.6) | 0.391 |
| MPO, 4 h, ng/ml | 23.5 (11.8)* | 24.6 (9.2)* | 22.6 (8.7)* | 0.456 |
| TNF-α, 0 h, pg/ml | 5.2 (4.1) | 5.1 (3.7) | 4.7 (3.6) | 0.415 |
| TNF-α, 4 h, pg/ml | 9.6 (4.1)* | 9.5 (4.1)* | 9.7 (3.9)* | 0.562 |
| CRP, 0 h, μg/ml | 0.9 (1.3) | 0.9 (1.1) | 0.8 (1.2) | 0.432 |
| CRP, 4 h, μg/ml | 1.4 (3.1) | 1.8 (2.2) | 1.3 (2.6) | 0.643 |
Values are mean ± (SD).
p < 0.05 compared to time respective point 0 h.
(sRAGE, soluble receptor for advanced glycation end products; IL-6, interleukin-6; MPO, myeloperoxidase; TNF-α, tumor necrosis factor alpha; CRP, C-reactive protein).
When patients were divided according to their use (n = 16) or not use (n = 9) of CBP we found no difference in all measured cytokines and biomarker (IL-6, TNF-α, sRAGE, S100A12, MPO, CRP) at baseline 0 h and at 4 h after surgery between patients in the OPCAB group compared to patients undergoing CABG with CPB (Table 2). This suggests that the use of cardiopulmonary bypass does not by itself contributes independently to the up-regulation of S100A12 and sRAGE as previously suggested by the positive association of CPB time with postoperatively S100A12 and sRAGE [10].
The mean hospital length of stay until discharge was 7.2 days and there was no difference between the groups treated by CABG with CPB and treated by OPCAB (Table 1). Interestingly, when we stratified all 25 patients undergoing coronary artery bypass grafting according to their hospital length of stay (LOS), we found that patients hospitalized 7 days or more (n = 13 including 5 patients with OPCAB) had higher 4h postoperative levels for S100A12 and sRAGE compared to patients with LOS ≤ 6 days (n = 12 including 4 patients with OPCAB; Figure 1). However, no significant difference was observed in 4 h postoperative levels of IL-6, TNF-α, MPO, and CRP between both groups. Notably, at baseline, patients with LOS ≥ 7 days had higher levels of TNF-α (6.3 pg/ml ± 3.3 vs 3.4 pg/ml ± 4.2, p = 0.002) but other marker including sRAGE, S100A12, IL-6, MPO, or CRP did not differ among patients with LOS ≥ 7 and LOS ≤ 6. Moreover, there was no significant difference in all clinical parameter including age, gender, smoking status, LV-function, diabetes hypertension, statin use and COPD between patients with LOS ≥ 7 days or LOS ≤ 6 days. Importantly, the additive Euro-Score, an index o f co-morbidities that correlates with mortality after cardiothoracic surgery [11], was similar for patients with LOS ≥ 7 (7.9, range 5-13) and LOS ≤ 6 days (8.3, range 6-12). Our finding of a positive association of postoperatively elevated S 100A12 and sRAGE plasma concentration with length of hospitalization in adults undergoing non-urgent coronary artery bypass grafting extents earlier studies demonstrating a positive correlation of S100A12 and/or sRAGE with the onset of acute lung injury and adult respiratory distress syndrome [7,10,13]. Our study suggests that both markers could be considered as useful early biomarker to indicate postoperative complications after cardiac surgery.
Figure 1.
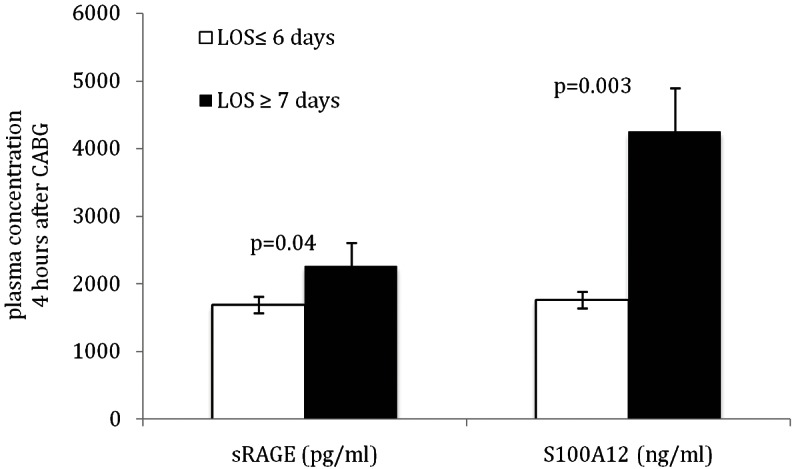
Level of plasma S100A12 and sRAGE measured 4 h after coronary artery bypass surgeries are significantly elevated in patients with prolonged hospital length of stay (LOS).
Discussion
In this prospective pilot study measuring plasma sRAGE and S100A12 in adult patients undergoing coronary artery bypass grafting we observed an association of 4 h postoperatively elevated S100A12 and sRAGE plasma levels with length of hospitalization. This confirms Liu et al.’s report that showed a strong correlation of postoperatively increased S100A12 (p < 0.001) and sRAGE levels (p = 0.012) with impaired lung function, acute lung injury and hospital LOS in young children undergoing cardiac surgery for congenital heart disease [10]. Importantly, while Liu’s study found a correlation of those biomarker with the time spent on CPB suggesting that contact with non-endothelial surfaces present in CPB could possibly augment the release of S100A12 from neutrophils, we did not observe any difference in S100A12 or sRAGE with regards to the use of CPB in patients undergoing coronary artery grafting bypass surgery. Plasma abundance of S100A12 might stem from activated neutrophils, since S100A12 composes up to 5% of neutrophil cytosolic protein and is secreted upon activation of protein kinase C or released during neutrophil damage. In addition of serving as a biomarker, an association of plasma S100A12 with the length of hospitalization after CABG surgery might potentially indicate a direct role of S100A12 in modulating inflammatory pathways associated with postoperative complications. This is supported by our recent findings examining S100A12 expression in aortic smooth muscle. We found that pathological expression of S100A12 in the medial layer of aneurysmal aortic tissue predicted the length of hospitalization in patients undergoing surgical repair of thoracic aortic aneurysms [14]. Furthermore, we reported on a co-localization of S100A12 with caspase-3 in the aortic media, and found that reduction of S100A12 in cultured human aortic smooth muscle cells harvested from aneurysms attenuated cell inflammation and apoptosis. This supports the hypothesis that S100A12 is not only is a biomarker of inflammation, but also may direct participate in modulating inflammation.
Our study has limitations that includes the small size of only 25 patients being studied, and non-randomization to the different treatment strategies. There is considerable discussion as to whether the use of CPB impacts systemic inflammation in patients undergoing coronary artery bypass grafting, with many, although mostly small studies like ours favor a reduction in systemic inflammation by avoiding CBP. This is reviewed by Raja et al [4]. Although in our single center study we found a similar length of hospitalization between OPCAB and CABG with CPB, others have shown a significant shorter duration in the length of hospitalization in a prospectively randomized trial comparing both surgical techniques [15]. Two randomized large clinical trials examining the benefits of CABG without cardiopulmonary bypass were recently reported. The study by Diegeler et al. found no difference in composite outcome of death, stroke, myocardial infarction, repeat revascularization, or new renal-replacement therapy within 30 days and within 12 month after surgery in 2539 patients 75 years and older randomized to either off-pump CABG or on-pump CABG [16]. A second large multicenter randomized clinical trial examined the quality of life, neurocognitive function and clinical outcome 1 year after randomization of 4752 patients undergoing CABG on pump or off pump, and found no significant difference in the rate of the primary or composite outcome [17].
Taken together, our study confirms and extends the findings of Liu et al. and suggests that the trauma related to cardiothoracic surgery itself, rather than intrinsic factors specific to CPB may mediate the marked increase in serum S100A12 and sRAGE measured 4 hours after cardiac surgery; however larger studies are needed for confirmation. Moreover, although suggested by our pilot study, larger studies are needed to examine whether S100A12 and sRAGE could serve as useful biomarker to predict postoperative complications in patients undergoing cardiac surgery.
Acknowledgments
This study was supported by funding from the Doris Duke Charitable Foundation. Dr. Hofmann Bowman is a recipient of the Doris Duke Clinical Scientist Development Award.
Disclosure of conflict of interest
All authors have reported that they have no relationship relevant to the contents of this paper to disclose.
References
- 1.Downing SW, Edmunds LH Jr. Release of vasoactive substances during cardiopulmonary bypass. Ann Thorac Surg. 1992;54:1236–1243. doi: 10.1016/0003-4975(92)90113-i. [DOI] [PubMed] [Google Scholar]
- 2.Laffey JG, Boylan JF, Cheng DC. The systemic inflammatory response to cardiac surgery: implications for the anesthesiologist. Anesthesiology. 2002;97:215–252. doi: 10.1097/00000542-200207000-00030. [DOI] [PubMed] [Google Scholar]
- 3.Roth-Isigkeit A, Hasselbach L, Ocklitz E, Bruckner S, Ros A, Gehring H, Schmucker P, Rink L, Seyfarth M. Inter-individual differences in cytokine release in patients undergoing cardiac surgery with cardiopulmonary bypass. Clin Exp Immunol. 2001;125:80–88. doi: 10.1046/j.1365-2249.2001.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raja SG, Berg GA. Impact of off-pump coronary artery bypass surgery on systemic inflammation: current best available evidence. J Card Surg. 2007;22:445–455. doi: 10.1111/j.1540-8191.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- 5.Hofmann Bowman MA, Schmidt AM. S100/calgranulins EN-RAGEing the blood vessels: implications for inflammatory responses and atherosclerosis. Am J Cardiovasc Dis. 2011;1:92–100. [PMC free article] [PubMed] [Google Scholar]
- 6.Meijer B, Gearry RB, Day AS. The role of S100A12 as a systemic marker of inflammation. Int J Inflam. 2012;2012:907078. doi: 10.1155/2012/907078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wittkowski H, Sturrock A, van Zoelen MA, Viemann D, van der Poll T, Hoidal JR, Roth J, Foell D. Neutrophil-derived S100A12 in acute lung injury and respiratory distress syndrome. Crit Care Med. 2007;35:1369–1375. doi: 10.1097/01.CCM.0000262386.32287.29. [DOI] [PubMed] [Google Scholar]
- 8.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–955. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Chen Q, Shi S, Shi Z, Lin R, Tan L, Yu J, Shu Q, Fang X. Plasma sRAGE enables prediction of acute lung injury following cardiac surgery in children. Crit Care. 2012;16:R91. doi: 10.1186/cc11354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurki TS, Jarvinen O, Kataja MJ, Laurikka J, Tarkka M. Performance of three preoperative risk indices; CABDEAL, EuroSCORE and Cleveland models in a prospective coronary bypass database. Eur J Cardiothorac Surg. 2002;21:406–410. doi: 10.1016/s1010-7940(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann Bowman MA, Heydemann A, Gawdzik J, Shilling RA, Camoretti-Mercado B. Transgenic expression of human S100A12 induces structural airway abnormalities and limited lung inflammation in a mouse model of allergic inflammation. Clin Exp Allergy. 2011;41:878–889. doi: 10.1111/j.1365-2222.2011.03714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jabaudon M, Futier E, Roszyk L, Chalus E, Guerin R, Petit A, Mrozek S, Perbet S, Cayot-Constantin S, Chartier C, Sapin V, Bazin JE, Constantin JM. Soluble form of the receptor for advanced glycation end products is a marker of acute lung injury but not of severe sepsis in critically ill patients. Crit Care Med. 2011;39:480–488. doi: 10.1097/CCM.0b013e318206b3ca. [DOI] [PubMed] [Google Scholar]
- 14.Das D, Gawdzik J, Dellefave-Castillo L, McNally EM, Husain A, Raman J, Hofmann Bowman MA. S100A12 expression in thoracic aortic aneurysm is associated with increased risk of dissection and perioperative complications. J Am Coll Cardiol. 2012;60:775–785. doi: 10.1016/j.jacc.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Ruzzeh S, George S, Bustami M, Wray J, Ilsley C, Athanasiou T, Amrani M. Effect of off-pump coronary artery bypass surgery on clinical, angiographic, neurocognitive, and quality of life outcomes: randomised controlled trial. BMJ. 2006;332:1365. doi: 10.1136/bmj.38852.479907.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diegeler A, Börgermann J, Kappert U, Breuer M, Böning A, Ursulescu A, Rastan A, Holzhey D, Treede H, Rieß FC, Veeckmann P, Asfoor A, Reents W, Zacher M, Hilker M GOPCABE Study Group. Off-Pump versus On-Pump Coronary-Artery Bypass Grafting in Elderly Patients. N Engl J Med. 2013;368:1189–1198. doi: 10.1056/NEJMoa1211666. [DOI] [PubMed] [Google Scholar]
- 17.Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, Straka Z, Piegas LS, Akar AR, Jain AR, Noiseux N, Padmanabhan C, Bahamondes JC, Novick RJ, Vaijyanath P, Reddy SK, Tao L, Olavegogeascoechea PA, Airan B, Sulling TA, Whitlock RP, Ou Y, Pogue J, Chrolavicius S, Yusuf S CORONARY Investigators. Effects of Off-Pump and On-Pump Coronary-Artery Bypass Grafting at 1 Year. N Engl J Med. 2013;368:1179–1188. doi: 10.1056/NEJMoa1301228. [DOI] [PubMed] [Google Scholar]


