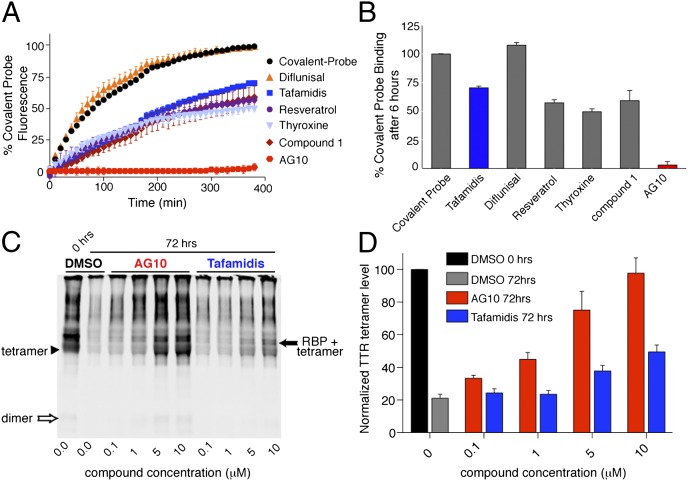
Fig. 3.
AG10 binds selectively to TTR in human serum. (A) Fluorescence change caused by modification of TTR in human serum by covalent probe 3 monitored for 6 h in the presence of probe alone (black circles) or probe and TTR ligands (colors). (B) Percentage of covalent probe binding to TTR in the presence of ligands measured after 6 h of incubation relative to probe alone. (The lower the binding of the probe, the higher the binding selectivity of the ligand to TTR.) Each bar shows the mean (SD) of three replicates. (C and D) Stabilization of WT-TTR in serum against acid-mediated denaturation in the presence of AG10 and tafamidis. Serum samples were incubated (with DMSO, AG10, or tafamidis) in acetate buffer (pH 4.0) for the desired time period (0 and 72 h) before cross-linking and immunoblotting. The intensity of TTR bands (TTR tetramer, arrowhead; TTR bound to RBP, solid arrow) was quantified by using an Odyssey IR imaging system (LI-COR Bioscience) and reported as percentage of TTR tetramer, calculated as 100 × [(tetramer and tetramer + RBP density, 72 h)/(tetramer and tetramer + RBP density of DMSO, 0 h)]. (C) The shown blot is representative of four independent blots. (D) Error bars indicate SEM (n = 4).