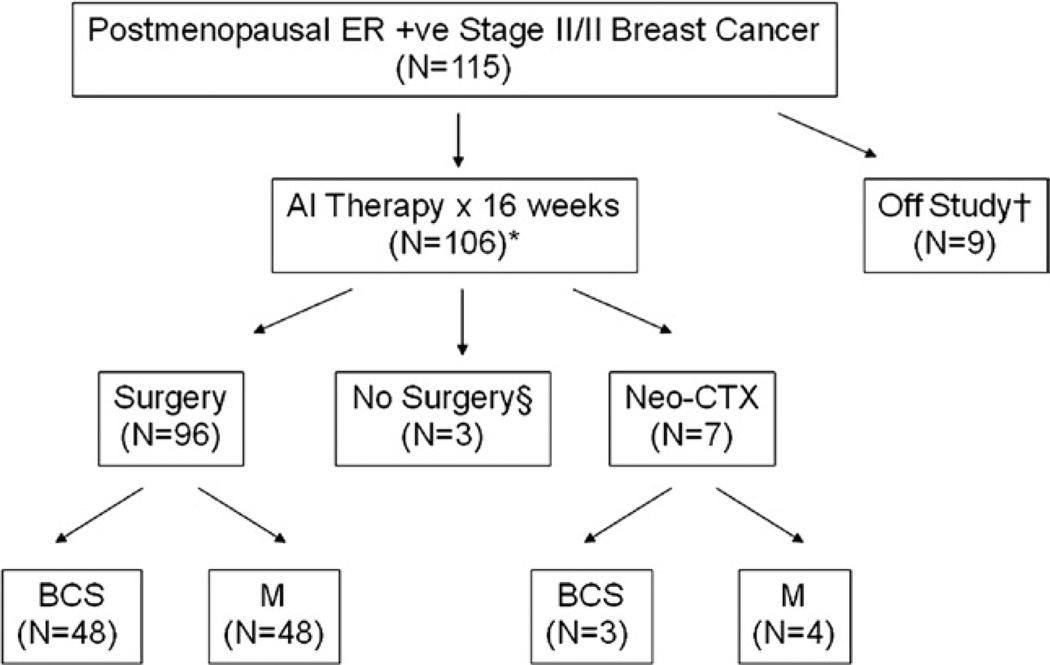
Figure 1.
This Consolidated Standards of Reporting Trials (CONSORT)–type diagram summarizes patient progress though the protocol. †Six patients withdrew consent, two patients were ineligible on additional investigation and one patient died before receipt of study drug. *Fourteen patients were treated on a protocol defined extension to 24 weeks for patients experiencing a partial response at 16 weeks who were thought to potentially benefit from additional tumor regression. §One patient refused operation and two patients did not have operations because of severe intercurrent illness unrelated to study drug. AI, aromatase inhibitor; BCS, breast-conserving surgery; CTX, chemotherapy treatment; ER, estrogen receptor; M, mastectomy.