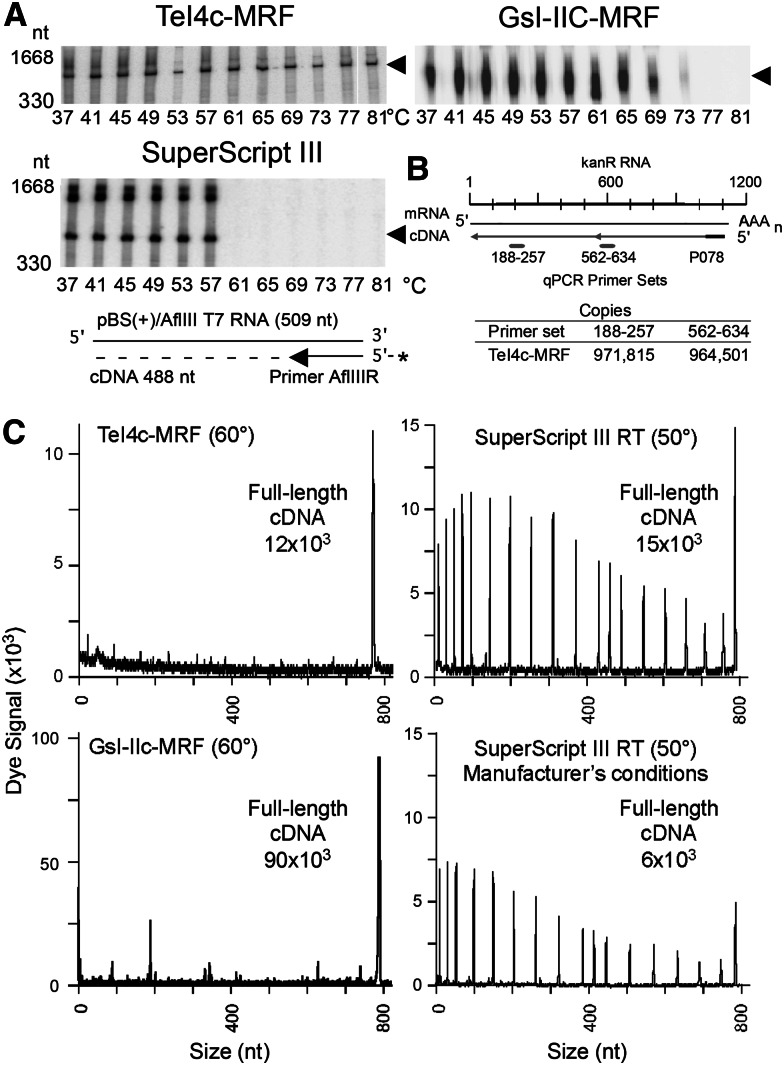
FIGURE 2.
Thermostability and processivity of group II intron RTs. (A) Gel assays of cDNA synthesis at different temperatures. A 509-nt in vitro–transcribed RNA (pBluescript KS(+)/AflIII) with a 5′-32P-labeled (star) primer (AflIIIR) annealed near its 3′ end was incubated for 30 min with TeI4c-MRF (2 μM), GsI-IIC-MRF (200 nM), or SuperScript III (10 units/μL) RTs, and the products were analyzed in a denaturing 6% polyacrylamide gel. Arrowheads to the right of the gel indicate the position of full-length cDNAs, and numbers to the left indicate positions of size markers (10-bp ladder). The regions of the gels containing the labeled DNA primer are shown in Supplemental Figure S2. (B) Taqman qRT-PCR. A 1.2-kb kanR RNA with primer P078 annealed near its 3′ end was reverse transcribed with TeI4c-MRF (200 nM) for 30 min at 60°C. The Table shows cDNA copies detected with primer sets 188–257 and 562–634, which detect cDNAs of 920 and 546 nt, respectively. (C) Capillary electrophoresis assays of cDNA synthesis. An 807-nt in vitro transcript containing an Ll.LtrB-ΔORF group II intron RNA with a fluorescently labeled DNA primer (5′ fluorophore WellRED) annealed near its 3′ end was reverse transcribed for 30 min with TeI4c-MRF RT (1 μM), GsI-IIC-MRF RT (200 nM), or SuperScript III (10 units/μL). cDNA lengths were determined relative to fluorescently labeled DNA markers (data not shown).