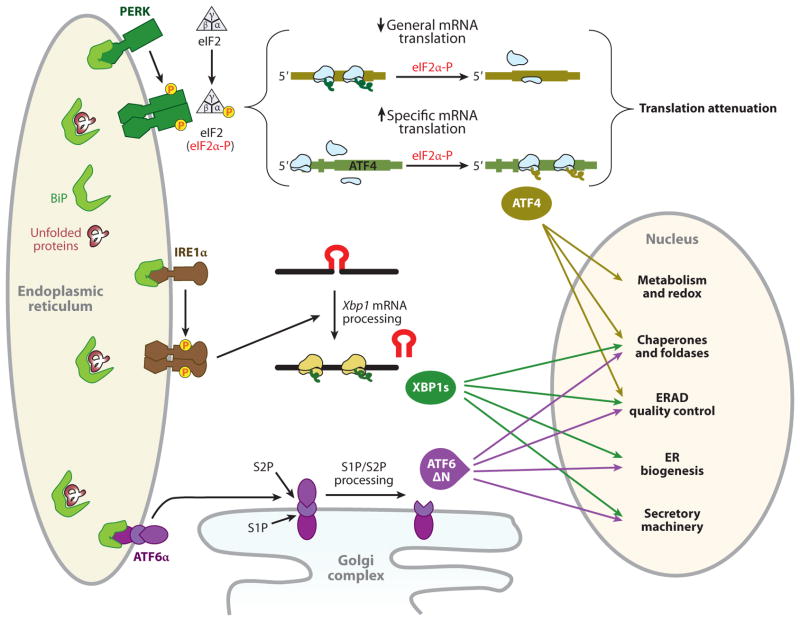
Figure 1.
The adaptive unfolded protein response (UPR). Activation of three UPR pathways initiates the adaptive endoplasmic reticulum (ER) stress response. During activation of the UPR in mammals, BiP (immunoglobulin heavy chain binding protein, also known as GRP78) is sequestered through binding to unfolded or misfolded polypeptide chains, thereby leading to BiP release from the ER stress sensors for their activation. Unconventional cytoplasmic splicing, mediated by IRE1α, removes a 26-nucleotide intron from unspliced X-box-binding protein 1 (Xbp1) mRNA (encoding 267 amino acids) to produce a translational frameshift, yielding a fusion protein encoded from two evolutionarily conserved open reading frames (16). The fusion protein, XBP1s, acts as a potent transcription factor for expression of UPR target genes involved in protein folding and export from the ER, export and degradation of misfolded proteins, and lipid biosynthesis, to resolve ER stress (16). Upon accumulation of unfolded protein in the ER lumen, oligomerization of the PKR-like ER kinase (PERK) in ER membranes induces its autophosphorylation and kinase domain activation (141, 142). Activated PERK phosphorylates serine 51 on the α-subunit of heterotrimeric eIF2 (143). When eukaryotic translation initiation factor 2α (eIF2α) is phosphorylated, the eIF2 complex shows increased affinity for its guanine nucleotide exchange factor eIF2B and sequesters all available eIF2B. Because the cellular level of eIF2B is 10- to 20-fold lower than the level of eIF2, very small changes in eIF2α phosphorylation can dramatically change the rate of translation initiation (144). Inhibition of general mRNA translation by the phosphorylation of eIF2α reduces accumulation of misfolded protein in the ER lumen (22), thereby protecting the cell from diverse stimuli that perturb the ER homeostasis. In contrast to inhibition of general mRNA translation, the PERK/eIF2α pathway stimulates the translation of several specific mRNAs containing multiple 53-upstream open reading frames, such as Atf4 and Atf5, Chop, Gadd34, and the cationic amino acid transporter 1 (Cat-1, an Na+-independent transporter of L-arginine and L-lysine) (28, 145). Among them, ATF4 activates transcription of the adaptive genes that encode functions in ER protein folding, endoplasmic reticulum–associated degradation (ERAD), amino acid biosynthesis and transportation, and the antioxidative stress response (24). Under ER stress, ATF6α and ATF6βare released from BiP and translocate to the Golgi complex, where they are cleaved by Golgi-resident proteases, first by S1P (site 1 protease) and then in the intramembrane region by S2P (site 2 protease), to release the N-terminal basic leucine zipper protein (bZIP) transcription factor domain (16). The bZIP domain of ATF6α then translocates into the nucleus, where it activates the transcription of genes encoding ER-localized molecular chaperones and folding enzymes, ERAD, protein secretion machineries, and ER biogenesis (146), in some cases in cooperation with XBP1s (42).