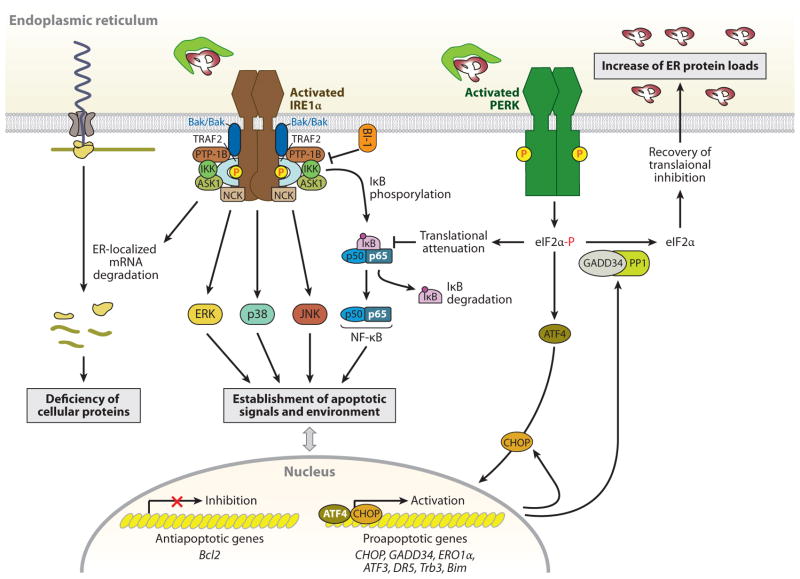
Figure 2.
IRE1α- and PERK-mediated cell death pathways. During endoplasmic reticulum (ER) stress, inositol-requiring protein 1α (IRE1α) forms a hetero-oligomeric complex with TNF receptor-associated factor 2 (TRAF2) (76) and apoptosis signal-regulating kinase 1 (ASK1) (77) and then recruits the protein kinase JNK, leading to the activation of JNK (76). The IRE1α-TRAF2 complex recruits IκB kinase (IKK), which phosphorylates inhibitor of κB (IκB), leading to the degradation of IκB and the nuclear translocation of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) (147). IRE1α also modulates the activation of other “alarm genes,” such as p38 and ERK (80), possibly by the binding of the Src homology (SH) 2/3 -containing adaptor proteins Nck and TRAF2, respectively. Furthermore, several proapoptotic (i.e., BAX/BAK, AIP1, and maybe PTP-1B) or antiapoptotic proteins (i.e., BI-1) interact with IRE1α, regulating its activation state (81–84). Thus, the formation of a macromolecular signaling complex of IRE1α with several proapoptotic proteins can generate apoptotic signals and establish an apoptotic environment. In addition, the endoribonuclease activity of IRE1α, aside from specific cleavage of Xbp1 mRNA, degrades ER-targeted mRNAs that can decrease cellular functions, such as proinsulin synthesis in β-cells. In contrast to the adaptive response by the PERK-phosphorylated eIF2α-ATF4 pathway, this pathway also contributes to stress-induced cell death by ATF4-mediated induction of proapoptotic genes, including CHOP, ATF3, and GADD34 (16). The induced transcription factor CHOP contributes to increased expression of the proapoptotic factors, such as death receptor 5 (DR5) (148), tribbles-related protein 3 (Trb3) (149), and binding to microtubule (Bim) (150), and it can suppress B cell lymphoma 2 (Bcl2) expression. Bim is also activated through protein phosphatase 2A-mediated dephosphorylation, which prevents its ubiquitination and proteasomal degradation (150). PERK-mediated translational attenuation upregulates NF-κB-dependent transcription because IκB has a shorter half-life than NF-κB, so NF-κB is released to translocate to the nucleus (151). Recovery from translational repression is mediated by eIF2α dephosphorylation by the two regulatory subunits of protein phosphatase 1 (PP1), GADD34 and CReP (constitutive repressor of eIF2α phosphorylation) (16). GADD34 is induced transcriptionally during ER stress by ATF4, whereas CreP is a constitutive activator of PP1. The premature dephosphorylation of eIF2α by the GADD34-PP1 complex restores translation of general mRNAs, which may be detrimental if the ER protein-folding defect is not resolved.