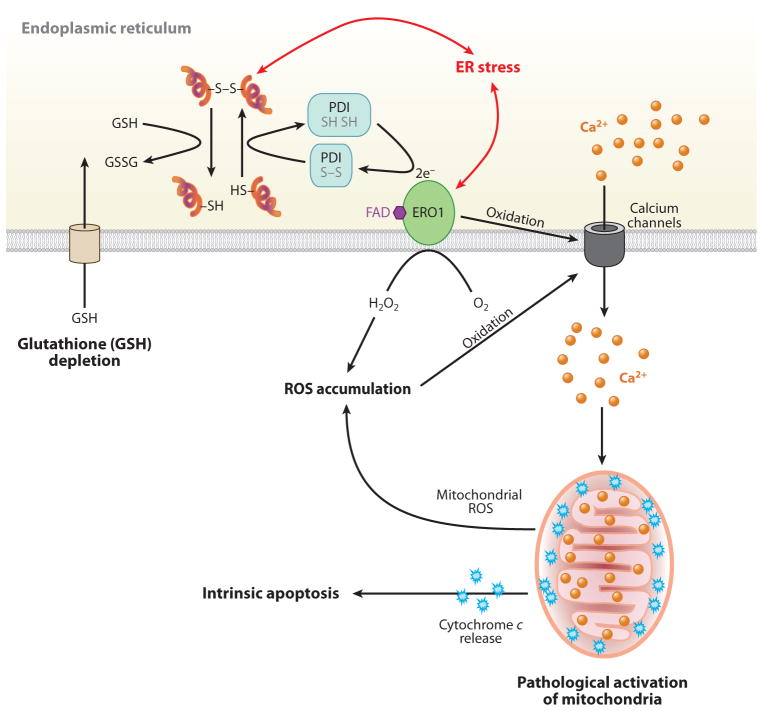
Figure 3.
The role of calcium and reactive oxygen species (ROS) in endoplasmic reticulum (ER) stress-mediated cell death. In the ER lumen, oxidative protein folding is catalyzed by protein disulfide isomerase (PDI) and ER oxidoreductase (ERO1). In this reaction, an oxidant flavin adenine dinucleotide (FAD)-bound ERO1 oxidizes PDI, which subsequently oxidizes folding proteins directly. FAD-bound ERO1 then passes two electrons to molecular oxygen, resulting in the production of hydrogen peroxide (73). During unfolded protein response activation, CHOP-mediated induction of ERO1α hyperoxidizes the ER lumen and causes oxidation-induced activation of the ER Ca2+ release channel inositol 1,4,5-trisphosphate receptor (152), causing a large and transient release of Ca2+ from the ER. Increased cytosolic Ca2+ is taken up into the mitochondrial matrix, and this stimulates mitochondrial ROS production through disruption of mitochondrial electron transport (153). High levels of ROS generated from mitochondria, in turn, further increase Ca2+ release from the ER. The increase in mitochondrial Ca2+ eventually dissociates cytochrome c from the inner membrane cardiolipin, which triggers permeability transition pore opening and cytochrome c release across the outer membrane. Now the vicious cycle of ER calcium release and mitochondrial ROS production activates cytochrome c-mediated apoptosis. In addition, ER stress may cause consumption of excessive cellular glutathione (GSH) because reduced GSH may also assist in reducing nonnative disulfide bonds in misfolded proteins, resulting in the production of oxidized glutathione (GSSG).