Table 1.
Rh2(S-DOSP)4-Catalyzed Cyclopropenation of Pyridotriazoles with Alkynes
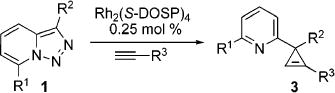
| no. | R1 | R2 | R3 | yield,a % | ||
|---|---|---|---|---|---|---|
| 1 | Cl | Ph | 1a | Ph | 3a | 81 |
| 2 | Cl | Ph | 1a | p-OMeC6H4 | 3b | 79 |
| 3 | Cl | Ph | 1a | p-CO2MeC6H4 | 3c | 65 |
| 4 | Br | Ph | 1b | Ph | 3d | 88b |
| 5 | Cl | p-OMeC6H4 | 1c | Ph | 3e | 67 |
| 6 | Cl | p-OMeC6H4 | 1c | o-tolyl | 3f | 45 |
| 7 | Cl | p-CF3C6H4 | 1d | Ph | 3g | 68 |
| 8 | Cl | p-CF3C6H4 | 1d | 1-cyclohexenyl | 3h | 93 |
| 9 | Cl | CO2Me | 1e | p-tolyl | 3i | 93c |
| 10 | Cl | CO2Me | 1e | p-OMeC6H4 | 3j | 67 |
| 11 | Cl | CO2Me | 1e | p-CO2MeC6H4 | 3k | 72 |
| 12 | Cl | CO2Me | 1e | m-CO2MeC6H4 | 3l | 87 |
| 13 | Br | Ph | 1b | n-butyl | 3m | 69 |
| 14 | Cl | CO2Me | 1e | Ph | 3n | 86d |
| 15 | Cl | Ph | 1a | (CH2)3Cl | 3o | 68 |
Isolated yield.
8% ee.
86% ee.
84% ee.
