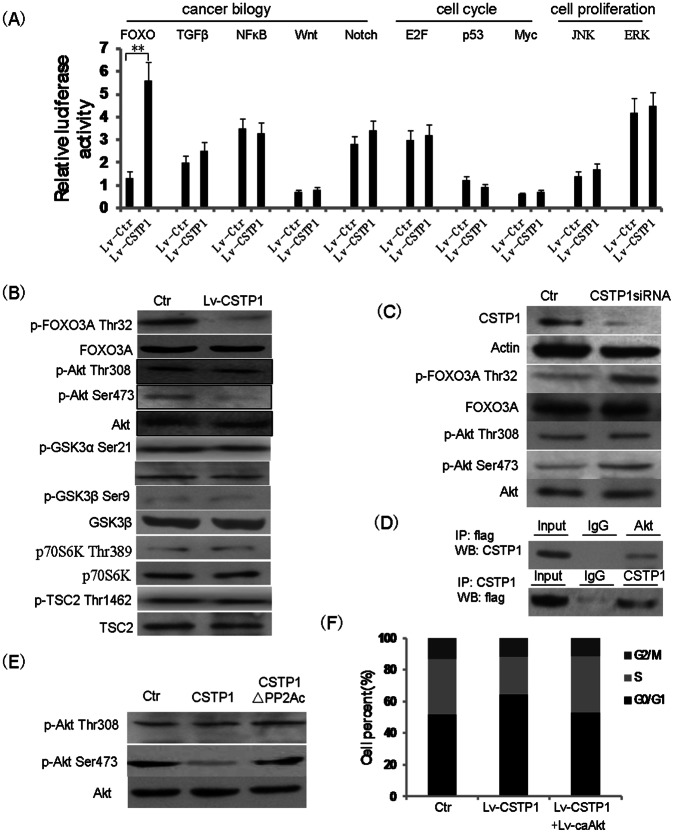
Figure 7. CSTP1 interacts with and dephosphorylates Akt at Ser473 site.
(A) EJ cells overexpressing CSTP1 and control cells were transfected with a series of cignal reported plasmid, 48 h later, luciferase activity was measured using the luciferase assay system with a luminometer. luciferase-based reporter signal is normalized to the expression of a cotransfected Renilla luciferase control plasmid. (B) Western blot analysis of the phosphorylation levels of FOXO3A, Akt,GSK3α, GSK3β, p70S6K and TSC2 in EJ cells overexpressing CSTP1. Unphosphorylated FOXO3A, Akt, GSK3α, GSK3β, p70S6K and TSC2 proteins were detected as internal controls. (C) Western blot analysis of the phosphorylation levels of FOXO3A and Akt after knocking down of CSTP1 in SV-HUC1 cells. (D) CSTP1 interacts with Akt in 293T cells. 293T cells were cotransfected with pcDNA3.1-CSTP1 and Flag-Akt expressing plasmids, interaction between CSTP1 and Akt was analyzed by co-ip experiment with anti-Flag (up panel) or anti-CSTP1 antibody(low panel). (E) Dephosphorylation of Akt by purified CSTP1 in vitro. Pure His-Akt was incubated with GST-tagged CSTP1 or GST-tagged CSTP1ΔPP2Ac in the phosphatase buffer. For a negative control, CSTP1 protein was omitted from the reaction mixture(Ctr). (F) EJ cells were overexpressed CSTP1 (Lv-CSTP1) or CSTP1(Lv-CSTP1) plus phosphor-mimetic S473D construct of Akt (ca-Akt) by lentivirus, cell cycle was analyzed by FACS. All experiments were performed at least three times.