Abstract
Background
Breast cancer is the leading cause of malignancy among women. Screening using mammography is proposed as an effective intervention for reducing early deaths due to breast cancer. We conducted a systematic review to assess the cost-effectiveness of such screening programs. We searched Medline, Scopus and Google Scholar and complemented it by other searches using sensitive search terms from 1993–2010. We screened the titles and abstracts, assessed the full texts of the remaining studies, and extracted data to a pre-designed data extraction sheet. Studies were categorized according to the age groups of the target population. We used narrative synthesis approaches for analyzing the data. Twenty-eight articles met the minimum inclusion criteria, mostly from high income settings. All studies used secondary data, and a variety of modeling techniques, age groups, screening intervals and outcome measures. Cost per life year gained, ranging from $1,634 (once at the age of 50 in India) to $65,000 (extending the lower age limit of screening to 40 Australian study), was the most commonly used outcome measure. Biennial screening test for those aged 50–70 years seems to be the most cost-effective option ($2685). Biennial screening for aged 50–70 years is the most cost-effective option among alternative scenarios. Screening those aged less than 50 is not recommended. Further studies in low-income and middle-income countries, and cost effectiveness studies along with randomized trials are required. To improve the comparability of the findings, future studies should include biennial screening in 50–70 age groups as an alternative strategy.
Keywords: Cost effectiveness, Breast cancer, Screening, Systematic review
Introduction
Breast cancer, with an incident rate of 1.1 million per year, is the leading cause of malignancy among women. The condition is responsible for the death of 410000 individuals worldwide (1–3). Amid the considerable increase in its global incident rate, reports have noted the increase to be more considerable in low-income and middle-income countries (LMICs) (4). In 2002 about half of women diagnosed with breast cancer were from LMICs, and the disease was considered as the most prevalent malignancy among female in the Middle East (5–7).
The progressive increase in the incidence of the disease along with the reduced quality of life and the high cost of medical care have imposed a heavy burden on the health systems and the society as a whole. It has been argued that effective use of screening programs is an efficient method for reducing early death due to breast cancer (8,9). The adoption of national mammography screening programs in many countries including in Europe, North America, Australia and Japan is a result of conducting randomized controlled trials of mammography screening that demonstrated a decline in deaths from breast cancer (10). On the other hand recent systematic reviews of randomized controlled trials of breast cancer screening programs have cast doubt on the effectiveness of such interventions (11). Still such screening programs are conducted, or are being introduced in several countries.
The current wisdom in many countries is to weigh the costs of new interventions against their benefits before implementing a new program (12–14). Analyzing the cost effectiveness is a useful tool in comparing different strategies and in calculating the costs and effectiveness of the strategies in reducing mortality or disease rate (15). Considering the limited resources and equity concerns for health systems, the importance of making decisions about healthcare interventions based on cost effectiveness evidence is increasing every day.
Cost effectiveness analysis can provide experts with useful information for programming and developing a breast cancer control policy. Such an analysis can provide data required for balancing the budget and fair allocation of the limited resources in national breast cancer control programs and subsequently determining the most effective way for providing diagnostic and therapeutic care (16). With a limited number of primary research studies originating from LMICs, systematic reviews of available evidence are valuable assets for decision making in such settings (17, 18).
We systematically reviewed the studies on cost effectiveness of breast cancer screening using mammograms. Our study updates a systematic review that was published in 1993 (19). We found no other comparable study since 1993. Our interest in conducting this review originated from heated debates among policy makers in Iran (health system and other sectors) about the program, many in favor of a national screening program. We aimed to provide a summary of the best available evidence to support policy makers, especially in countries where local evidence does not suffice, in making informed decision about conducting breast cancer screening and identifying the target population for screening as well as the screening intervals.
Methods
Study Design
Systematic review of cost effectiveness studies of breast cancer screening using mammograms. We included studies that incorporated cost-effectiveness studies alongside randomized controlled trials, or used modeling techniques to estimate cost-effectiveness ratios.
Inclusion Criteria
The articles studying the cost effectiveness of breast cancer screening using mammograms in women’s normal population were included. We included cost-effectiveness studies alongside trials as well as those studies that used modeling techniques based on routine data or data from previous studies.
The systematic reviews studying the cost effectiveness of breast cancer screening using mammograms were eligible for inclusion if they answered the same question as this study. The studies, whose results were used in the already added systematic reviews, however, were excluded.
Studies on mammogram techniques (comparing different mammogram technologies), studies of screening in women with special diseases, studies of other screening approaches (e.g. self-examination, physical examination), studies with ambiguous target age groups, and studies of opportunistic screening approaches were not included.
Search strategy
We systematically searched international databases of Cochrane, Pubmed, Scopus, Science Direct, and Google Scholar web pages from January 1993 March 2010, limited to English language publications.
A previous systematic review of similar topic had been published in 1993 (19). In that study they systematically searched for literature up to 1990, and included papers published until late 1992 (20). We searched for studies published after Brown et al. (1993) review, and for studies published 1990–1993 and not already included in that study. We also searched the reference lists of the identified studies.
In order to find all the related articles, we used sensitive search strategies to reduce the risk of losing any articles. Briefly “breast cancer + screening” was used to search the Cochrane Library and “(economic evaluation OR cost effectiveness OR cost utility OR burden of disease) AND (breast cancer)” to search other databases.
Selecting studies, quality assessment and data extraction
The titles of the resulted articles were entered in the data management software and the duplicates were eliminated. We then screened the titles of identified papers and excluded those that were obviously unrelated to our review. All the abstracts of the remaining articles were assessed for inclusion by four authors. The studies that we all agreed that they were unrelated to our study were omitted at this stage. The full texts of the remaining articles were assessed for final inclusion in the study. Two authors independently assessed the quality of included studies using Drummond’s et al tool. One author extracted data from included studies into the data extraction sheet and it was checked by another author.
Grouping and analysis
The included articles were then divided into groups based on assessing the cost effectiveness of breast cancer screening in different target age groups:
1- Women aged between 50 and 70
2- Women aged over 70
3- Women aged below 50
4- Studies of women of other age groups not matching the above criteria (e.g. 40–69 years)
Thereafter, the findings of the included studies were outlined in specifically designed data extraction sheets.
We also noted the outcome measures reported in the studies. Our main outcome measure was the cost per life year saved, although other outcome measures such as cost per Quality Adjusted Life Years (QALY) gained, cost per death averted, cost per Disability Adjusted Life Years (DALY) averted, and cost per cancer detected were also considered.
Only data reporting the incremental cost effectiveness ratio of the screening methods using mammograms compared with not doing any intervention were considered for the final analysis. The incremental cost effectiveness ratio calculated through comparing different screening interventions were excluded. The costs reported for each event were similarly entered in the study if the price was mentioned in US dollars; as for those mentioned in other currencies, conversion was made based on the year the study was accepted for publication. In cases when the acceptance date was not clear, the exchange rate at the time of publication was used.
Results
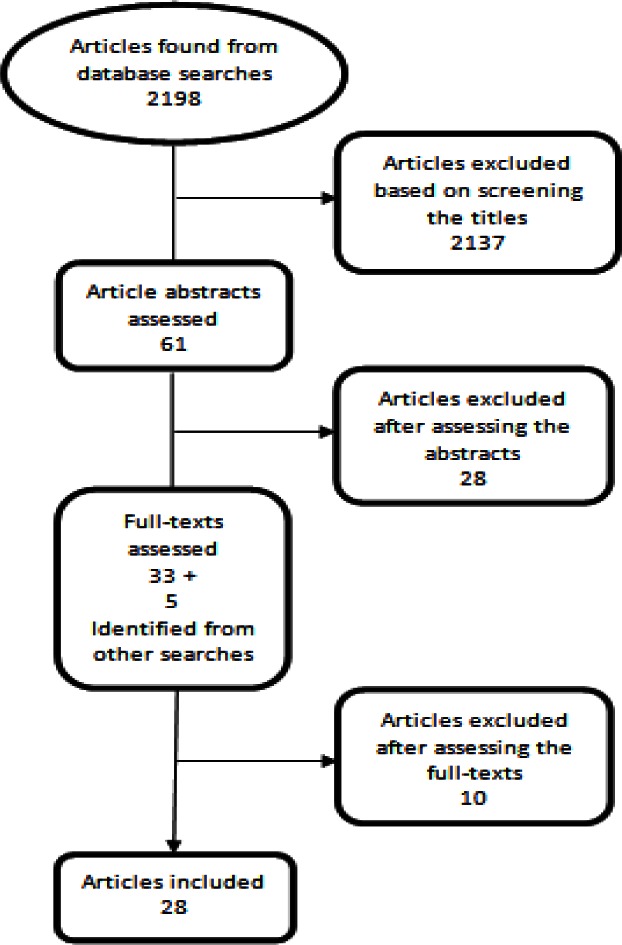
Our searches yielded 2198 hits. After initial screenings and assessments, we assessed the full-texts of 38 studies and included 28 studies (Fig. 1). These included 26 primary studies and two systematic reviews. During the process we also identified and excluded six non-systematic reviews (21–26) and one systematic review (27) that was concerned with comparing a screening program with an existing or alternative screening program.
Fig. 1:
Summary of the search and article selection process for cost-effectiveness studies of mammography screening
General description of the included studies
The cost effectiveness of breast cancer screening has mainly been assessed in high income countries, with only one such study being conducted in a LMICs: India (28) [1]. Other studies were conducted in China’s Hong Kong (29, 30) [2], the US (19, 31–35) [6], the Netherlands (36–38) [3], Australia (20, 39, 40) [3], Finland (41, 42) [2], Norway (43, 44) [2], the UK (45, 46) [2], France (47) [1], Germany (48) [1], Switzerland (49) [1], New Zealand (50) [1], Spain (51) [1], Korea (52) [1] and Slovenia (53) [1].
The included studies used a variety of modeling techniques: Markov modeling [7], MIcrosimulation SCreening ANalysis (MISCAN) [7] and MICROIFE a similar microsimulation model based on MISCAN [1], Decision trees [5], discrete event simulation [1], stochastic mathematical simulation [2] and simple modeling technique [3]. The majority of the studies [18] followed a healthcare system perspective and assessed only the program’s direct costs. Eight studies, however, took a societal perspective (Table 1). The most common cost effectiveness measure used in the studies was cost per life years saved, calculated for alternatives based on age groups and screening intervals. Other measures used in the studies included: cost per QALY gained, cost per DALY averted, cost per cancer detected, cost per death averted and per life saved. The majority of the studies extracted the data on the quality and efficacy of the screening tests from the available ongoing screening programs, local cancer registries and routine data. We identified no cost effectiveness study conducted alongside a prospective interventional study.
Table 1:
The number of identified primary studies of breast cancer screening using mammography in each age group and their main methodological characteristics*
| Age groups | Number of studies* | Perspective | Outcome measures | Modeling approach |
|---|---|---|---|---|
| 50–70 | 18 | Health system [14], societal [4] | Life year saved [14], QALY [6], death averted [3], cancer detected [2], DALY [1], life saved [1] | MISCAN [6], Markov [4], Decision tree [2], Simple modeling [3], Mathematical simulation [1], Discrete event simulation [1], MICROLIFE [1] |
| Over 70 | 1 | Health system [1] | Life year saved [1] | Markov [1] |
| Below 50 | 2 | Health system [2] | Life year saved [1], QALY [1] | Markov[1], Decision tree [1] |
| different age groups | 11 | Health system [7], societal [4] | Life year saved [8], QALY [2], death averted [1], cancer detected [1], DALY [1] | MISCAN [3], Markov [4], Decision tree [2], MICROLIFE [1], Stochastic [1] |
The papers add up to more than 28 papers, as a few papers provide data under different age groups
The quality assessment results showed that from the 26 primary studies, only one study was of low quality [35], and was excluded from further analysis.
Eighteen studies were assessed to be of high quality and seven had medium quality levels. All studies conducted after 2000 were assessed as having high quality, suggesting the methodological rigor and reporting standards had improved through the time.
Cost-effectiveness of mammography screening
We classified each article under one or more groups based on the age group of the target population. Four studies were classified in more than one group (28, 31, 39, 50).
50–70 age group (Table 2)
Table 2:
Summary findings of studies of cost effectiveness of breast cancer screening in women aged between 50 and 70 years. All the studies used secondary data for modeling
| Author, year | Country | Perspective | Model | Intervals, age groups | Results |
|---|---|---|---|---|---|
| VanIneveld et al., 1993 (36) | Different countries | Health system | MISCAN | Biennial 50–70 years | Cost per life year gained for Netherlands, UK, France and Spain, respectively: ≈$3162, ≈$2685, ≈$8651 and ≈$14468. |
| Carter et al., 1993 (39) | Australia | Health system | MISCAN | Biennial 50–69 years, triennial 50–69 years | Cost per life year gained, respectively: $14733 and $13081 |
| Rosenquist et al., 1994 (31) | USA | Health system | Markov | Annual 60–69 years | $15,500 per life year gained |
| Beemsterboer et al., 1994 (48) | Germany | Health system | MISCAN | Biennial 50–69 years | ≈$11135 per life year gained and ≈$11728 per QALY gained. |
| Szeto et al., 1996 (50) | New Zealand | Health system | MICROLIFE | Biennial 50–64 years, triennial 50–64 years, biennial 50–69 years | Cost per life year gained, respectively: $14510, $12668, $14,597. |
| Plans et al., 1996 (51) | Spain | Health system | simple modeling | 50–64 years (interval not determined) | $8424 per cancer detected |
| Hakama et al., 1997 (41) | Finland | Societal | simple modeling | Biennial 50–69 years | Cost per death averted, per life year gained and per QALY gained, respectively: $77100, $15400 and $15900 |
| Salzmann et al., 1997 (32) | USA | Health system | Markov | Annual 50–69 years, biennial 50–69 | Cost per life year gained, respectively: $45700 and $21400; cost per QALY gained, respectively: $46500, $21700 |
| Boer et al., 1998 (45) | UK | Health system | MISCAN | Triennial 50–64 years, triennial 50–69 years, biennial 50–64 years | Cost per life year gained, respectively: ≈$4195, ≈$4343, ≈$4506; cost per death averted, respectively: ≈$41824, ≈$40265, ≈$46353 |
| Leivo et al., 1999 (42) | Finland | Societal | simple modeling | Biennial 50–59 years | $18955 per life year gained |
| Norum, 1999 (43) | Norway | Health system | Decision tree | Biennial 50–69 years | Cost per cancer detected and cost per life year gained, respectively: ≈$17202 and ≈$14208. |
| Wang et al., 2001 (44) | Norway | Health system | Decision tree | Biennial 50–69 years | Cost per life year gained, and cost per life saved respectively: $3750 and $86045 |
| Arveux et al., 2003 (47) | France | Health system | Markov | Annual 50–65 years | $25000 per life year gained |
| Groot et al., 2006 (37) | Different countries | Societal | Mathematical simulation | Biennial 50–70 years | Cost per DALY averted, respectively for Africa, North America and Asia: $75, $915 and $75. |
| Stout et al., 2006 (33) | USA | Societal | Discrete event simulation | 5-yearly 55–70 years | $27000 per QALY gained |
| Okonkwo et al., 2008 (28) | India | Health system | MISCAN | Once at age 50, biennial 50–70 years | Cost per life year gained, respectively: $1634 and $3308; cost per death averted: $22220 and $36731 |
| Rojnik et al., 2008 (53) | Slovenia | Health system | Markov | Triennial 50–65 years | ≈$9801 per QALY gained |
| de Gelder et al., 2009 (49) | Switzerland | Health system | MISCAN | Biennial 50–69 years | Cost per life year gained and per QALY gained, respectively: ≈$16895 and ≈$18233. |
Most studies have focused on mammography screening in this age group. A previously systematic review published in 1993 found that the cost-effectiveness range was from $3,400 to $83,830 per life years saved (19). We included 18 articles targeting this age group in this review. Apart from a study conducted in India (28), the others were performed in high income countries. MISCAN (six studies) was the commonest modeling techniques used in the studies (28, 36, 39, 45, 48, 49). The cost per life years saved ranged from $1634 (screening once at the age of 50 in India (28)) to $45700 (annual screening age 50–69 in the US (32)). While studying the routine screening techniques, a multi-country study of the cost effectiveness of biennial screening at age 50–70 in Britain, with $2685 per life years saved, was the most cost effective option (36). The cost per QALY gained ranged from $9801 (triennial screening at age 50–65 in Slovenia (53)) to $46500 (annual screening at age 50–69 in the US (32)). In a study conducted in the Netherlands in 2006 (37), cost per DALY averted in Africa, North America and Asia was estimated at $75, $915 and $75, respectively. Compared with the other studies, this latter study revealed a considerably lower costs per DALY saved, indicating that the accuracy and generalizability of the estimated costs and DALYs averted might be questionable.
Based on the evidence provided here, among continuous screening programs, a biennial screening from 50–70 seems to be the most cost-effective option.
‘Over 70’ and ‘less than 50’ age groups (Table 3)
Table 3:
Summary of studies of cost effectiveness of breast cancer screening in women aged over 70 or below 50. All the studies used secondary data for modeling
| Author, year | Country | Perspective | Model | Intervals, age groups | Results |
|---|---|---|---|---|---|
| Rosenquist et al., 1994 (31) | USA | Health system | Markov | Annual 80–85 years | $35000 per life year gained |
| Annual 40–49 years | $26200 per life year gained | ||||
| Biennial 40–49 years | $14000 per life year gained | ||||
| Madan et al., 2010 (46) | UK | Health system | Decision tree | Triennial 47–49 | ≈$44692 per QALY gained |
For those aged over 70, a study from the US reported the cost per life years saved of $35000 for annual screening of those aged between 80 and 85 compared with no screening (31). In a former systematic review (40), the cost of per QALY gained by expanding the upper limit of screening from 50–69 to 79 ranged from $8119 to $27751.
In those aged less than 50, two studies from the US and Britain were identified (31, 46). The cost per life years saved, from annual and biennial screening of those aged 40–49 was $26200 and $14000, respectively. Barratt et al (40) had reported that starting the screening from age 40 instead of 50 would cost 24000 to 65000 US dollars per QALY gained. Moreover, the cost per QALY gained for triennial screening those aged 47 to 49 was about $45000 (46).
Based on the evidence provided here, mammography screening does not seem to be cost-effective in age groups under 50 or over 70.
Other age groups (Table 4)
Table 4:
Summary of studies of cost effectiveness of breast cancer screening in women of other age groups. All the studies used secondary data for modeling
| Author, year | Country | Perspective | Model | Intervals, age groups | Results* |
|---|---|---|---|---|---|
| Hall et al., 1992 (20) | Australia | Health system | Decision tree | Biennial 45–69 years | $7190 |
| Carter et al., 1993 (39) | Australia | Health system | MISCAN | Annual 40–49 plus biennial 50–69; Biennial 40–49 plus triennial 50–69; Biennial 40–69 |
$ 27257 $19919 $20300 |
| Rosenquist et al., 1994 (31) | USA | Health system | Markov | Annual 40–85; Annual 50–85 |
$18600 $16800 |
| Lindfors et al., 1995 (34) | USA | Health system | Markov | Seven different age group scenarios | From $16000 to $31900 |
| Szeto et al., 1996 (50) | New Zealand | Health system | MICROLIFE | Biennial 45–64 | $15169 |
| Rosenquist et al., 1998 (35) | USA | Societal | Markov | Four different age group scenarios | From $16100 to $18800 |
| Woo et al., 2007 (29) | Hong Kong | Societal | Decision tree | Biennial 50–74; Biennial 40–74; Annual 50–74; Annual 40–74 |
$90771, $107310, $321608, $385092, per DALY averted |
| Wong et al., 2007 (30) | Hong Kong | Societal | Markov | Biennial 40–69 | $64400, or $61600 per QALY gained |
| Okonkwo et al., 2008 (28) | India | Health system | MISCAN | Once at age 40; Biennial 40–60 |
$6496, and $3468; or $110542 and $46021 per death averted |
| Lee et al., 2009 (52) | Korea | Health system | Stochastic | Triennial 45–65 | $100007 per cancer detected |
Reported costs are “per life year gained” unless otherwise specified.
Ten studies were included in this group. The cost per life years saved ranged from $3468 (biennial screening in India at age 40–60 (28)) to $64400 (biennial screening in China’s Hong Kong at age 40–69 (29)). The only study that reported cost per QALY gained, reported it at $61600 per QALY gained in a biennial screening at 40–69 in China’s Hong Kong (30). As a result, it seems that little evidence exists for other age groups.
Studies from LMICs
The Indian study, which was the only study conducted in a LMIC, compared four strategies: once at 50, biennial screening from 50 to 70, once at 40, and biennial screening from 40 to 60 (28). The study concluded that the cost per life years saved of using each of these techniques was $1634, $3308, $6496 and $3468, correspondingly. The cost per death averted was $22220, $36731, $110542, and $46021, respectively. All the strategies reported in this research seemed more cost-effective compared with similar interventions reported in other studies. In summary, based on Okonkwo et al study (2008) the most cost-effective option is screening once at age 50, followed by biennial screening 50–70. However, the evidence on cost-effectiveness of mammography from LMICs is rare.
Discussion
The results suggest that biennial screening for breast cancer using mammograms conducted on individuals aged between 50 and 70 years might be the most cost effective option in many parts of the world. It may also be concluded that screening individuals aged more than 70 is less cost effective than those aged between 50 and 70. Despite the discrepancies between the results of different studies, the results also suggest that screening those aged less than 50 should not be recommended.
An important proportion of the discrepancies in cost-effectiveness estimates can be contributed to the observed variations in intervention designs and modeling approaches. Different studies used different effect estimates, including the number of life years saved, deaths prevented, and cancerous cases detected along with the number of QALYs gained and DALYs averted. The inconsistency could also be contributed to the country of the study, the age group of the target population and the age at which the screening had started. These differences reduce the chance of comparing the results of different studies conducted in this field.
The cost per life year saved, which ranged between $1600 (one time screening at the age of 50 in India (28)) to $65000 (expanding screening coverage from the minimum age of 50 to 40 (40)), was the most commonly used outcome measure. Moreover, the cost per QALY gained reported in the studies ranged from $8100 (extending the upper limit of the screening age from 69 to 79 (40)) to $61600 (biennial screening in those aged 40–69 in China’s Hong Kong (30)).
Brown et al’s systematic review in 1993 only included studies of screening in women aged over 49 that reported a direct measure of the outcome such as cost per life year saved and cost per death averted (19). They reported a wide range of cost effectiveness estimates (cost per life year saved from $3400 to $83900). Similarly our review estimated the cost per life year saved of continuous screening programs from $2700 to $64400. In that sense, although recent studies have increased the evidence base for decision-making, they have not changed the bottom line conclusions reached several years ago. They have, however, increased the range of the countries from which the studies are reported. Study perspective in economic evaluations is the viewpoint that establishes whose benefits are sought and whose costs are considered in weighing up the evidence. Choosing the ‘right’ perspective has a critical impact on estimating the costs and outcomes of an intervention (15). Depending on the characteristics of the setting and the interventions, a change in the perspective may affect the overall conclusions of the study. The majority of the studies included in our review had adopted a health systems perspective. Future studies should pay more attention to societal aspects of conducting breast cancer screening programs.
We identified only one study that assessed the cost effectiveness of the breast cancer screening programs in a LMIC (28). Formulating a relevant national policy for implementation in a country requires evidence from settings similar to that country (18). The cost effectiveness of breast cancer screening depends on the demographic and epidemiologic characteristics of the country, the incidence rate of breast cancer, patients care seeking behavior and the number of deaths from breast cancer before performing the screening, as well as the characteristics of the screening strategy such as the population’s compliance with the program, the target age group, and the interval between each screening test. Furthermore, the internal capacity and the infrastructure of a health system for conducting screening programs may affect the choice of the strategy as well as the outcomes of the program. Hence extrapolating the findings to LMICs should be conducted with care. Using the findings of this review, it seems a biennial screening test for those aged between 50 and 70 (the minimum observed costs of $2685 for per life year saved (36)) is the most cost effective option. Biennial screening in 50–70s has been cost efficient in India, with estimated cost per life year saved of $3300 (28). Other alternative strategies require more justification for being recommended for adoption in LMICs. It should not be assumed that all screening programs result in improvements in health outcomes (54). Hence, the limited evidence available to us does not justify making any concrete recommendations of using such screening programs. Also for LMICs, the capacity issues remain a challenge. Should such a program start as a national policy, or should it go through a region by region staged expansion in the country (55)? Starting a program at a national level may produce logistic and implementation issues, while it may provide political and public support that may be difficult to achieve for local pilots and staged expansions. The current evidence does not provide enough evidence to answer such questions, and policy maker should consider evidence from implementation studies when making decisions on how to adopt a screening program. As an example, it has been observed choosing the strategies that did not match the infrastructural capacity of LMICs has resulted in limited success in achieving outcomes expected from cervical cancer screening programs (56, 57). As such, it might be advisable that in LMICs, breast cancer screening programs should be conducted only as pilot programs alongside a comprehensive evaluation plan.
Conclusion
Our findings suggest a need for conducting further studies of cost-effectiveness of breast cancer screening. It seems that performing cost effectiveness studies along with clinical trials in LMICs might be useful. Moreover, using the evidence available so far, it seems prudent to recommend such studies to include the strategies that incorporate biennial screening in 50–70 age groups, while screening below the age of 50 is not recommended.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc) have been completely observed by the authors.
Acknowledgments
Our study was funded by Ministry of Health and Medical Education of Iran. The authors declare that there is no conflict of interest.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global Cancer Statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Breast Health Global Initiative Pan American Health Organization (Regional Office of the World Health Organization) 2010. Available from: http://www.paho.org/english/ad/bhgi-about.htm.
- 3.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 4.Okobia MN, Bunker CH, Okonofua FE, Osime U. Knowledge, attitude and practice of Nigerian women towards breast cancer: a cross-sectional study. World J Surg Oncol. 2006;4:11. doi: 10.1186/1477-7819-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahan E, Ibrahim AS, El Najjar K, Ron E, Al-Agha H, Polliack A, et al. Cancer patterns in the Middle East--special report from the Middle East Cancer Society. Acta Oncol. 1997;36(6):631–6. doi: 10.3109/02841869709001327. [DOI] [PubMed] [Google Scholar]
- 6.IARC . Cancer site by site. International Agency for Research on Cancer; Lyon: 2008. World Cancer Report 2008; pp. 412–418. [Google Scholar]
- 7.Harirchi I, Karbakhsh M, Kashefi A, Momtahen AJ. Breast cancer: results of a multi-center study. Asian Pac J Cancer Prev. 2004;5(1):24–27. [PubMed] [Google Scholar]
- 8.Shapiro S, Coleman EA, Broeders M, Codd M, de Koning H, Fracheboud J, et al. Breast cancer screening programmes in 22 countries: current policies, administration and guidelines. Int J Epidemiol. 1998;27(5):735–42. doi: 10.1093/ije/27.5.735. [DOI] [PubMed] [Google Scholar]
- 9.Maurer F. A peer education model for teaching breast self-examination to women. Cancer Nurs. 1997;20(1):49–61. doi: 10.1097/00002820-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 10.de Koning HJ. Mammographic screening: evidence from randomised controlled trials. Ann Oncol. 2003;14(8):1185–9. doi: 10.1093/annonc/mdg319. [DOI] [PubMed] [Google Scholar]
- 11.Gøtzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2009;4(1) doi: 10.1002/14651858.CD001877.pub3. [DOI] [PubMed] [Google Scholar]
- 12.Rajan A, Gutierrez-Ibarluzea I, Moharra M. Addressing issues assessment promotion: Motives, enablers, and barriers. Int J Technol Assess Health Care. 2011;27(01):55–63. doi: 10.1017/S0266462310001352. [DOI] [PubMed] [Google Scholar]
- 13.Hajarizadeh B, Rashidian A, Haghdoost AA, Alavian SM. Estimating the costs of the mass vaccination campaign against Hepatitis B in Iranian adolescents. Govaresh. 2011;14(1):27–34. [Google Scholar]
- 14.Soleymani F, Rashidian A, Dinarvand R, Kebriaeezade A, Hosseini M, Abdollahi M. Assessing the effectiveness and cost-effectiveness of audit and feedback on physician’s prescribing indicators: study protocol of a randomized controlled trial with economic evaluation. DARU Journal of Pharmaceutical Sciences. 2012;20:88. doi: 10.1186/2008-2231-20-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drummond M, Sculpher M, Torrance G, O’brien B, Stoddart G. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford: Oxford University Press; 2005. UK. [Google Scholar]
- 16.Radice D, Redaelli A. Breast cancer management: quality-of-life and cost considerations. Pharmacoecon. 2003;21(6):383–96. doi: 10.2165/00019053-200321060-00003. [DOI] [PubMed] [Google Scholar]
- 17.Yousefi Nooraie R, Rashidian A, Nedjat S, Majdzadeh R, Mortaz Hejri SO, Etemadi A, Salmasian H. Promoting development and use of systematic reviews in a developing country. J Eval Clin Pract. 2009;15(6):1029–34. doi: 10.1111/j.1365-2753.2009.01184.x. [DOI] [PubMed] [Google Scholar]
- 18.Rashidian A. Adapting valid clinical guidelines for use in primary care. Prim Care Respir J. 2008;2008;17(3):136–7. doi: 10.3132/pcrj.2008.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown M, Fintor L. Cost-effectiveness of breast cancer screening: preliminary results of a systematic review of the literature. Breast Cancer Res Treat. 1993;25(2):113–18. doi: 10.1007/BF00662136. [DOI] [PubMed] [Google Scholar]
- 20.Hall J, Gerard K, Salkeld G, Richardson J. A cost utility analysis of mammography screening in Australia. Soc Sci Med. 1992;34(9):993. doi: 10.1016/0277-9536(92)90130-i. [DOI] [PubMed] [Google Scholar]
- 21.Mushlin A, Fintor L. Is screening for breast cancer cost-effective? Cancer. 1992;69(S7):1957–62. doi: 10.1002/1097-0142(19920401)69:7+<1957::aid-cncr2820691716>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 22.Zappa M, Visioli C, Ciatto S. Mammography screening in elderly women: efficacy and cost-effectiveness. Crit Rev Oncol Hematol. 2003;46(3):235–39. doi: 10.1016/s1040-8428(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 23.Brown D, French M, Schweitzer M, McGeary K, McCoy C, Ullmann S. Economic evaluation of breast cancer screening: A review. Cancer Pract. 1999;7(1):28–33. doi: 10.1046/j.1523-5394.1999.07103.x. [DOI] [PubMed] [Google Scholar]
- 24.Elixhauser A. Costs of breast cancer and the cost-effectiveness of breast cancer screening. Int J Technol Assess Health Care. 1991;7(04):604–15. doi: 10.1017/s0266462300007169. [DOI] [PubMed] [Google Scholar]
- 25.Clark R. Economic issues in screening mammography. Am J Roentgenol. 1992;158(3):527. doi: 10.2214/ajr.158.3.1738989. [DOI] [PubMed] [Google Scholar]
- 26.Fraser N, Clarke P. Cost-effectiveness of breast cancer screening. Breast. 1992;1(4):169–72. [Google Scholar]
- 27.Mandelblatt J, Saha S, Teutsch S, Hoerger T, Siu A, Atkins D, et al. The Cost-Effectiveness of Screening Mammography beyond Age 65 Years: a systematic review for u.s preventive srvices. Ann Intern Med. 2003;139(10):835. doi: 10.7326/0003-4819-139-10-200311180-00011. [DOI] [PubMed] [Google Scholar]
- 28.Okonkwo QL, Draisma G, der Kinderen A, Brown ML, de Koning HJ. Breast cancer screening policies in developing countries: a cost-effectiveness analysis for India. J Natl Cancer Inst. 2008;100(18):1290–300. doi: 10.1093/jnci/djn292. [DOI] [PubMed] [Google Scholar]
- 29.Woo P, Kim J, Leung G. What is the most cost-effective population-based cancer screening program for Chinese women? J Clin Oncol. 2007;25(6):617. doi: 10.1200/JCO.2006.06.0210. [DOI] [PubMed] [Google Scholar]
- 30.Wong IO, Kuntz KM, Cowling BJ, Lam CL, Leung GM. Cost effectiveness of mammography screening for Chinese women. Cancer. 2007;110(4):885–95. doi: 10.1002/cncr.22848. [DOI] [PubMed] [Google Scholar]
- 31.Rosenquist C, Lindfors K. Screening mammography in women aged 40–49 years: analysis of cost-effectiveness. Radiol. 1994;191(3):647. doi: 10.1148/radiology.191.3.8184041. [DOI] [PubMed] [Google Scholar]
- 32.Salzmann P, Kerlikowske K, Phillips K. cost effectiveness of extending screening mammography guidlines to include women 40 to 49 years of age. Ann Intern Med. 1997;127:955–65. doi: 10.7326/0003-4819-127-11-199712010-00001. [DOI] [PubMed] [Google Scholar]
- 33.Stout NK, Rosenberg MA, Trentham-Dietz A, Smith MA, Robinson SM, Fryback DG. Retrospective cost-effectiveness analysis of screening mammography. J Natl Cancer Inst. 2006;98(11):774–82. doi: 10.1093/jnci/djj210. [DOI] [PubMed] [Google Scholar]
- 34.Lindfors K, Rosenquist C. The cost-effectiveness of mammographic screening strategies. JAMA. 1995;274(11):881. [PubMed] [Google Scholar]
- 35.Rosenquist C, Lindfors K. Screening mammography beginning at age 40 years A Reappraisal of Cost Effectiveness. Cancer. 1998;82(11):2235–40. doi: 10.1002/(sici)1097-0142(19980601)82:11<2235::aid-cncr19>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 36.van Ineveld B, van Oortmarssen G, de Koning H, Boer R, van der Maas P. How cost-effective is breast cancer screening in different EC countries? Eur J Cancer. 1993;29(12):1663–68. doi: 10.1016/0959-8049(93)90100-t. [DOI] [PubMed] [Google Scholar]
- 37.Groot M, Baltussen R, Uyl-de Groot C, Anderson B, Hortobágyi G. Costs and health effects of breast cancer interventions in epidemiologically different regions of Africa, North America, and Asia. Breast J. 2006;12(1):81. doi: 10.1111/j.1075-122X.2006.00206.x. [DOI] [PubMed] [Google Scholar]
- 38.Boer R, De Koning H, Van Oortmarssen G, Van Der Maas P. In search of the best upper age limit for breast cancer screening. Eur J Cancer. 1995;31(12):2040–43. doi: 10.1016/0959-8049(95)00457-2. [DOI] [PubMed] [Google Scholar]
- 39.Carter R, Glasziou P, Van Oortmarssen G, De Koning H, Stevenson C, Salkeld G, et al. Cost-effectiveness of mammographic screening in Australia. Aust J Pub Health. 1993;17(1) doi: 10.1111/j.1753-6405.1993.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 40.Barratt AL, Irwig LM, Glasziou PP, Salkeld GP, Houssami N. Benefits, harms and costs of screening mammography in women 70 years and over: a systematic review. Med J Aust. 2002;176(6):266–71. doi: 10.5694/j.1326-5377.2002.tb04405.x. [DOI] [PubMed] [Google Scholar]
- 41.Hakama M, Hristova L. Effect of screening in the Nordic cancer control up to the year 2017. Acta Oncol. 1997;36(2):119–28. doi: 10.3109/02841869709109219. [DOI] [PubMed] [Google Scholar]
- 42.Leivo T, Sintonen H, Tuominen R, Hakama M, Pukkala E, Heinonen O. The cost effectiveness of nationwide breast carcinoma screening, 1987–1992. Cancer. 1999;86(4):638–46. doi: 10.1002/(sici)1097-0142(19990815)86:4<638::aid-cncr12>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 43.Norum J. Breast cancer screening by mammography in Norway. Is it cost-effective? Ann Oncol. 1999;10(2):197–203. doi: 10.1023/a:1008376608270. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, Kåresen R, Hervik A, Thoresen S. Mammography screening in Norway: results from the first screening round in four counties and cost-effectiveness of a modeled nationwide screening. Cancer Causes Control. 2001;12(1):39–45. doi: 10.1023/a:1008999403069. [DOI] [PubMed] [Google Scholar]
- 45.Boer R, De Koning H, Threlfall A, Warmerdam P, Street A, Friedman E, et al. Cost effectiveness of shortening screening interval or extending age range of NHS breast screening programme: computer simulation study. BMJ. 1998;317(7155):376. doi: 10.1136/bmj.317.7155.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madan J, Rawdin A, Stevenson M, Tappenden P. A Rapid Response Economic Evaluation of the UK NHS Cancer Reform Strategy Breast Cancer Screening Program Extension via a Plausible Bounds Approach. Value Health. 2010;13(2):215–21. doi: 10.1111/j.1524-4733.2009.00667.x. [DOI] [PubMed] [Google Scholar]
- 47.Arveux P, Wait S, Schaffer P. Building a model to determine the cost-effectiveness of breast cancer screening in France. Eur J Cancer Care. 2003;12(2):143–53. doi: 10.1046/j.1365-2354.2003.00373.x. [DOI] [PubMed] [Google Scholar]
- 48.Beemsterboer P, De Koning H, Warmerdam P, Boer R, Swart E, Dierks M, et al. Prediction of the effects and costs of breast-cancer screening. Int J Cancer. 1994;58(5):623–28. doi: 10.1002/ijc.2910580502. [DOI] [PubMed] [Google Scholar]
- 49.de Gelder R, Bulliard JL, de Wolf C, Fracheboud J, Draisma G, Schopper D, et al. Cost-effectiveness of opportunistic versus organised mammography screening in Switzerland. Eur J Cancer. 2009;45(1):127–38. doi: 10.1016/j.ejca.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 50.Szeto K, Devlin N. The cost-effectiveness of mammography screening: evidence from a microsimulation model for New Zealand. Health Policy. 1996;38(2):101–15. doi: 10.1016/0168-8510(96)00843-3. [DOI] [PubMed] [Google Scholar]
- 51.Plans P, Casademont L, Salleras L. Cost-effectiveness of breast cancer screening in Spain. Int J Technol Assess Health Care. 1996;12(01):146–50. doi: 10.1017/s0266462300009478. [DOI] [PubMed] [Google Scholar]
- 52.Lee SY, Jeong SH, Kim YN, Kim J, Kang DR, Kim HC, et al. Cost-effective mammography screening in Korea: high incidence of breast cancer in young women. Cancer Sci. 2009;100(6):1105. doi: 10.1111/j.1349-7006.2009.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rojnik K, Naversnik K, Mateovic-Rojnik T, Primiczakelj M. Probabilistic cost-effectiveness modeling of different breast cancer screening policies in Slovenia. Value Health. 2008;11(2):139–48. doi: 10.1111/j.1524-4733.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 54.Omidvari AH, Vali Y, Murray S, Wonderling D, Rashidian A. Nutritional screening for improving professional practice for patient outcomes in hospital and primary care settings. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD005539.pub2. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murillo R, Díaz S, Sánchez O, Perry F, Pineros M, Poveda C, et al. Pilot implementation of breast cancer early detection programs in Colombia. Breast Care. 2008;3(1):29. doi: 10.1159/000114446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Denny L, Quinn M, Sankaranarayanan R. Chapter 8: Screening for cervical cancer in developing countries. Vaccine. 2006;24(3):S71–S77. doi: 10.1016/j.vaccine.2006.05.121. [DOI] [PubMed] [Google Scholar]
- 57.Alliance for Cervical Cancer Prevention . The Case for Investing in Cervical Cancer Prevention. Seattle: ACCP; 2004. Cervical Cancer Prevention Issues in Depth, No. 3. [Google Scholar]