Figure 3.
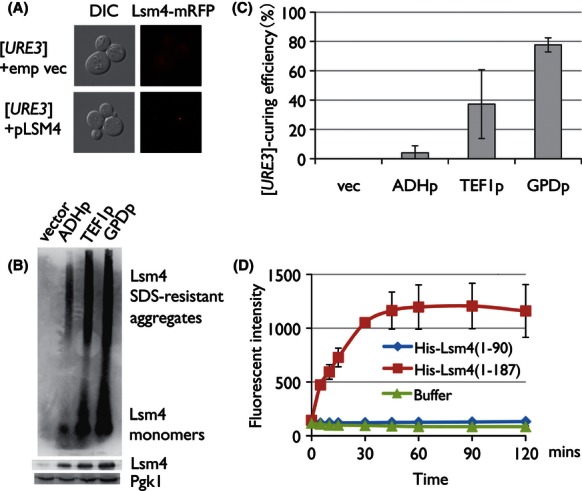
Lsm4 forms amyloid upon overexpression. (A) Emergence of Lsm4-mRFP foci on LSM4 overexpression. NPK302 was cotransformed with pRS415LSM4p-LSM4-mRFP (expressing LSM4-mRFP under the authentic promoter) and pRS425GPDp-LSM4 or an empty vector. Transformants were selected on SC-Leu-His, cultured to log phase, and subjected to fluorescence microscopy. (B) SDS-stable aggregate formation of Lsm4 in vivo. NPK302 was transformed with a pRS415-based plasmid to express the LSM4 gene under the control of the GPD (very strong), TEF1 (strong), or ADH (moderate) promoter, or an empty vector. Transformants were selected and cultured on SC-Leu, and subjected to the SDD-AGE experiment. NPK302 itself possesses no SDS-stable aggregate (data not shown). (C) Dose dependency of Lsm4's prion curability. The transformants selected in (B) were subjected to the colony color assay. (D) Amyloid formation of Lsm4 in vitro. 6xHis-tagged Lsm4 and Lsm41–90, which completely lacks the Q/N-rich region, were expressed in Escherichia coli using the pET15b expression system, and purified under a denaturing condition with 8 mol/L of urea, as previously described (Crist et al. 2003). The purified His-Lsm4 proteins were then subjected to the Thioflavin T-based amyloid formation assay. Lsm4 monomers were incubated in the Congo Red binding buffer (5 mmol/L potassium phosphate, pH 7.4, 150 mmol/L NaCl) with 20 μmol/L Thioflavin T using a revolution mixer (RVM-101, Asahi Techno Glass, Tokyo, Japan) in 30°C.
