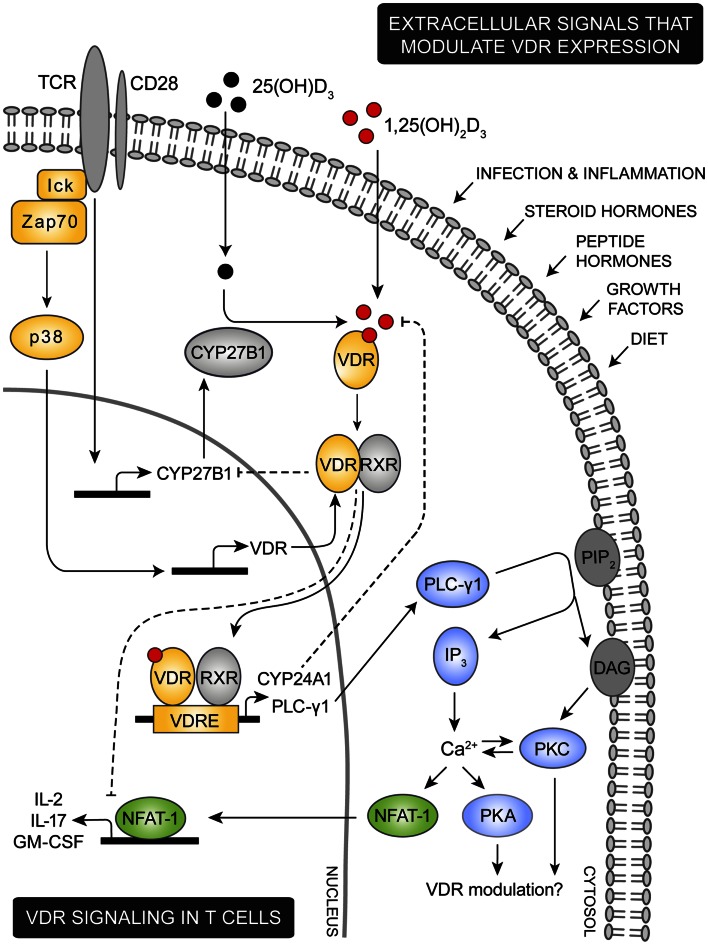
Figure 1.
Proposed model for VDR signaling in T cells. Various extracellular signals including infection, inflammation, steroid and peptide hormones, and diet are involved in regulation of the intracellular VDR level. During an immune response the TCR is triggered by specific antigens, inducing a cascade of intracellular signaling events. Among these, lck and ZAP-70 are activated leading to activation of the p38 kinase which in naïve human T cells induce expression of VDR. TCR triggering also promotes expression of the 1,25(OH)2D3 synthesis enzyme CYP27B1. Through intrinsic synthesis of 1,25(OH)2D3 and uptake of 1,25(OH)2D3 from the extracellular environment, VDR is activated and translocated into the nucleus where it either induce or suppress transcription of a variety of genes. As an example, VDR induce upregulation of PLC-γ1 in naïve human T cells. Once PLC-γ1 is expressed, TCR induced activation of PLC-γ1 leads to activation of PKA and PKC and an increase in the intracellular calcium level. In other cell types PKA and PKC has been shown to modulate expression of VDR, depending on the particular cell type and cellular differentiation state investigated. An increase in intracellular calcium concentration activates NFAT1 a necessary transcription factor for expression of IL-2 and other cytokines. IL-2 is a cytokine required for proliferation of T cells and one mechanism by which VDR adjust T cell activity is to outcompete NFAT1’s binding to the IL-2 promoter and furthermore to down-regulate the actual expression of NFAT1. To control VDR activity a series of negative feedback loops exists; activated VDR both induce expression of the 1,25(OH)2D3 degrading enzyme CYP24A1 and down-regulates expression of the 1,25(OH)2D3 synthesizing enzyme CYP27B1.