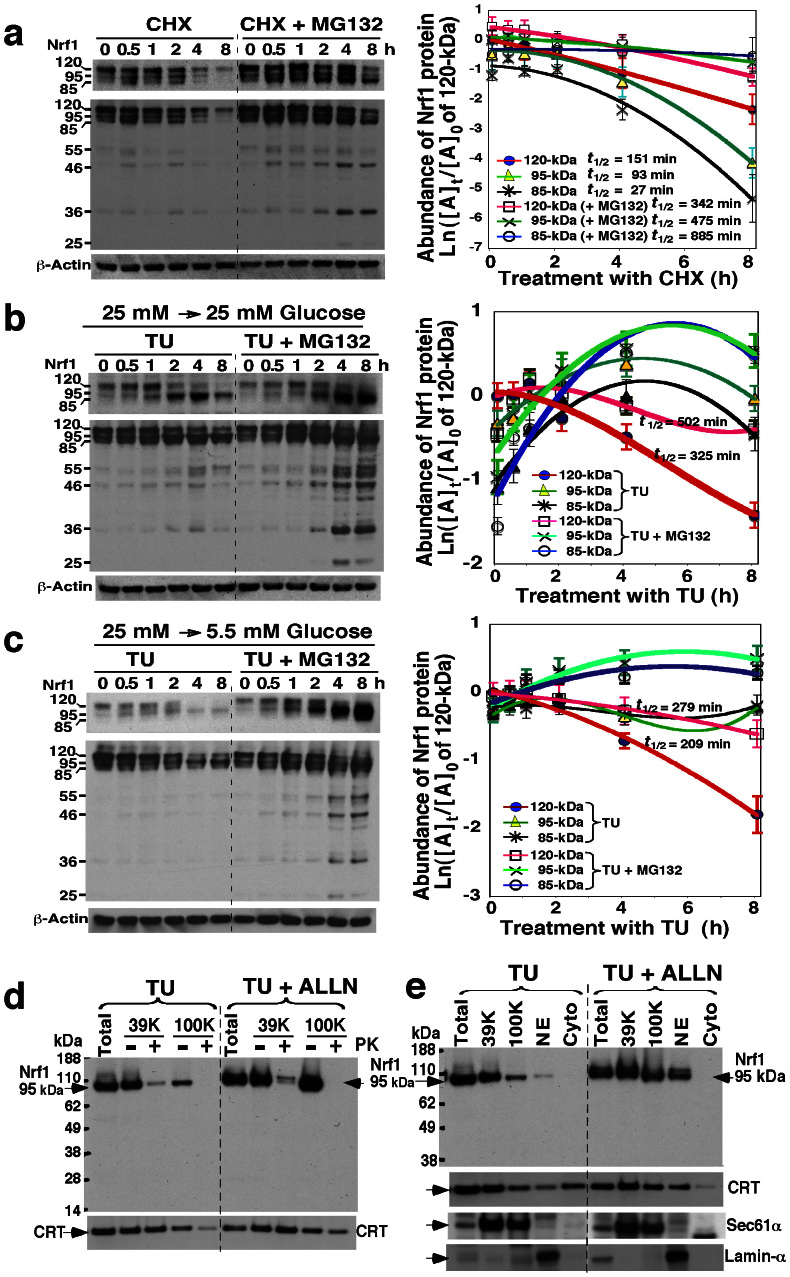
Figure 2. The stability of longer Nrf1 isoforms in response to changes in glucose concentration.
(a) Examination of the stability of Nrf1 isoforms. COS-1 cells were transfected with an Nrf1 expression construct for 6 h, and were allowed to recover from transfection for 16 h in the fresh 25 mM-glucose medium. The cells were treated with cycloheximide (CHX, 50 μg/ml) and/or MG132 (5 μM) for the indicated times before being disrupted. The abundance of Nrf1 proteins was determined by immunoblotting. The left upper shows a cropped image from the film that was exposed to the immunoblot for a shorter time to eliminate intense bands. The intensity of blots was calculated by dividing the values for Nrf1 with that for β-actin. The relative amount of the 120-, 95- and 85-kDa proteins at the indicated times ([A]t) was normalized to [A]0 (i.e. the value of 120-kDa at t0 after CHX), and is shown graphically (right, mean ± S.D, n = 4), along with their half-lives (t1/2). (b,c) Stability of Nrf1 isoforms in cells grown in 25 mM or 5.5 mM glucose medium upon blockage of glycosylation by tunicamycin (TU). Nrf1-expressing cells were transferred to 25 mM (b) or 5.5 mM (c) glucose medium, to which was added TU (1 μg/ml) and/or MG132 (5 μM) for the indicated times. Following western blotting, the relative abundance of the 120-, 95- and 85-kDa Nrf1 proteins was calculated after normalization to [A]0 of 120-kDa at t0 after TU and shown graphically (right, mean ± S.D, n = 4). (d,e) The non-glycosylated 95-kDa Nrf1 isoform is not protected by membranes. Nrf1-expressing cells were treated with TU (1 μg/ml) and/or ALLN (5 μg/ml) for 18 h in the fresh 25 mM-glucose medium, and then subjected to subcellular fractionation (e), followed immediately by membrane proteinase protection assays (d). The resulting products were identified by immunoblotting with V5 antibody. Calreticulin (CRT) was an ER-luminal marker, that existed in ER-enriched subcellular fractions purified by centrifuging at 39, 000 × g (39 K) and 100, 000 × g (100 K). Sec61α and Lamin-α were used to verify ER and nuclear envelope (NE) membranes.