Abstract
Neuropathic pain (NeP) is generally considered an intractable problem, which becomes compelling in clinical practice when caused by highly effective chemotherapeutics, such as in the treatment of cancer with oxaliplatin (OXA) and related drugs. In the present work we describe a structurally new compound, ADM_09, which proved to effectively revert OXA-induced NeP in vivo in rats without eliciting the commonly observed negative side-effects. ADM_09 does not modify normal behavior in rats, does not show any toxicity toward astrocyte cell cultures, nor any significant cardiotoxicity. Patch-clamp recordings demonstrated that ADM_09 is an effective antagonist of the nociceptive sensor channel TRPA1, which persistently blocks mouse as well as human variants of TRPA1. A dual-binding mode of action has been proposed for ADM_09, in which a synergic combination of calcium-mediated binding of the carnosine residue and disulphide-bridge-forming of the lipoic acid residue accounts for the observed persistent blocking activity toward the TRPA1 channel.
Pain is the evolved response to noxious stimuli1; it plays an important role in safeguarding the individual from potential sources of tissue destruction. However, the perception of pain can also be the result of a dysfunction of the nervous system. Typically, local injury or infections trigger the release of peripheral chemical mediators eliciting two diverse effects: (a) activate the inflammatory response attracting leukocytes to the point of injury, and (b) sensitize nociceptors (neurons responsible for the transmission of pain) enhancing their response to pain. Under some circumstances, however, nociceptors activity divorce from normal physiology and pain is produced in the absence of any appropriate stimulus. This is known as pathological pain or neuropathic pain (NeP). NeP can be caused by lesions to the central or peripheral nervous system, or can be induced by metabolic insult or trauma, autoimmune diseases, infections (e.g., HIV-1), diabetes, cancer, or it can be the consequence of the toxic side-effects of diverse drug regimens. From the behavioral point of view, NeP is a chronic algic sensation associated with altered sensibility, including allodynia (pain evoked by an innocuous stimulus) and hyperalgesia (amplified pain induced by a noxious stimulus), and with loss of functionality2,3,4. In states of NeP, the protective role of pain is lost, because the abnormal activity of nociceptive neurons does not offer biological advantage but just causes suffering and distress. Although nociceptors signal alteration is still a matter of debate, this abnormal activity of nociceptive neurons is thought to result from the increased neuronal expression and activation of ion channels and receptors that initiate and mediate the abnormal generation of action potentials and synaptic transmission in pain pathways5.
Therapeutically, NeP is considered an intractable problem. As a matter of fact, it is not readily treated with nonsteroidal anti-inflammatory drugs, whereas tricyclic antidepressants and antiepileptics are not particularly effective, while being associated with several negative side effects6. Conversely, opiates might be used for acute or chronic pains, e.g., in terminal cancer; yet, alleviation of NeP remains problematic because of the side-effects associated with opioid analgesia, such as opioid-like withdrawal and physical dependence, and because of the high doses often required, which reduce the gap between the effective and lethal dose (therapeutic index)7. These therapeutic limitations are particularly severe in commonly occurring situations, such as in the chronic NeP that is frequently observed in patients receiving antitumoral chemotherapy8. Treatment of this pain status is becoming compelling, since the development of new anticancer drugs will conceivably extend the survival of tumor-bearing hosts, so that neurotoxicity rather than tumor progression may become the treatment-limiting issue in the therapy of some kind of cancer9. At present, clinical use of highly effective chemotherapeutics, such as oxaliplatin, cisplatin, vincristine and paclitaxel, is severely compromised by the development of painful peripheral neuropathy, consisting of mechanical and cold allodynia, ongoing burning pain, tingling numbness, allodynic sensation in hands and feet, and chronic foot/leg, hand/arm numbness10. Although these chemotherapeutic agents have different neurotoxicity profiles, they all produce painful effects that are very often responsible for therapy interruption. It is thus clear that the availability of safe and effective analgesic drugs that may be used for the treatment of chronic NeP is a target of high interest and of urgent need11.
Acute and chronic neurotoxicities associated with the use of oxaliplatin (OXA) have been thoroughly described12,13. Recently, chronic OXA-induced neuropathy has been related to changes in the expression and sensitivity of transient receptors potential (TRP) and other cation channels14,15,16. In particular, the role of TRPA1 as a sensor of reactive compounds, such as those generated during oxidative stress caused by OXA and cisplatin administration, has been firmly established17. Furthermore, it has been reported that OXA-evoked allodynia and NeP in rats are mediated by TRPA1 and that channel activation is likely caused by glutathione-sensitive molecules15.
In this context, we describe herein an easily accessible, water-soluble derivative of lipoic acid, named ADM_09, which proved to be remarkably effective in the treatment of OXA-induced neuropathy without eliciting the most common negative side-effects. In our studies, we investigated the anti-oxidative properties of ADM_09 in vitro and in cellular systems, and we established the ability of ADM_09 to revert OXA-induced NeP in vivo. ADM_09 was also found devoid of any cardiotoxicity and of toxic effects toward astrocytes. Whole-cell patch clamp recordings demonstrated that ADM_09 is an effective antagonist of the TRPA1 receptor, showing inhibition activity in the low micromolar range; a mode of action fitting the experimental evidence has been proposed for the observed blocking activity.
Results
Synthesis
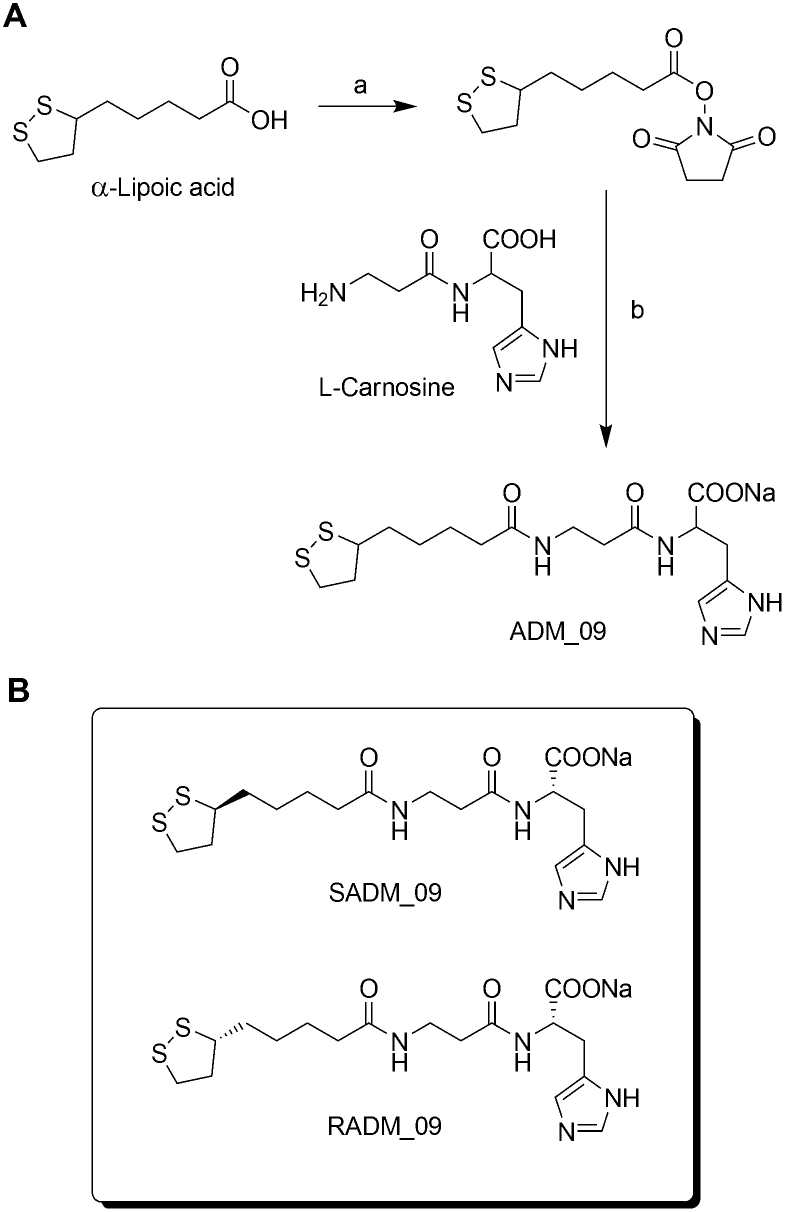
ADM_09 was synthesized in two steps from commercially available (±) α-lipoic acid and L-carnosine under very mild conditions (Fig. 1)18,19. The antioxidative and mild analgesic properties of lipoic acid are well documented20; for example, lipoic acid has been shown to reduce OXA-induced hyperlagesia in rats through anti-oxidative processes21. On the other hand, carnosine has been proven to scavenge reactive oxygen species (ROS) as well as α-β unsaturated aldehydes formed from peroxidation of cell membrane fatty acids during oxidative stress22. On this basis, the design of ADM_09 was aimed at achieving a synergic combination the two substrates through their incorporation into a single compound, with the goal of obtaining an enhancement of their antioxidative and analgesic properties. (±) α−Lipoic acid was activated by reacting with N-hydroxysuccinimide to form the corresponding ester, which was treated at room temperature with L-carnosine in the presence of sodium bicarbonate to afford ADM_09 in 73% overall yield as a crystalline sodium salt (Fig. 1).
Figure 1. Synthesis of ADM_09 carried out by coupling L-carnosine with (±)-α−Lipoic acid.
(A): synthetic scheme; (B): diastereomerically pure compounds obtained from L-carnosine and (+) and (−) (α)-lipoic acid. Reagents and conditions: a) N-hydroxysuccinimide, DCC, THF, r.t., 90%; b) NaHCO3, DMF/H2O 1:1, r.t., 81% (DCC = Dicyclohexylcarbodiimide, THF = tetrahydrofuran, DMF = dimethylformamide).
ADM_09 is a low molecular weight compound, soluble in water, stable under acidic (pH 1) and alkaline (pH 10) conditions, in saliva, and at room temperature and 37°C. ADM_09 did not show acute toxicity and fulfils the requirements of the so called “rule of five” for drug-likeness18,23.
To ascertain whether the two diastereomeric forms of ADM_09 would possess different biological activities, RADM_09 and SADM_09 were prepared as diastereomerically pure compounds by reacting L-carnosine with enantiomerically pure (R)-(+)- and (S)-(−)-α-lipoic acid, respectively (Fig. 1).
Antioxidative properties
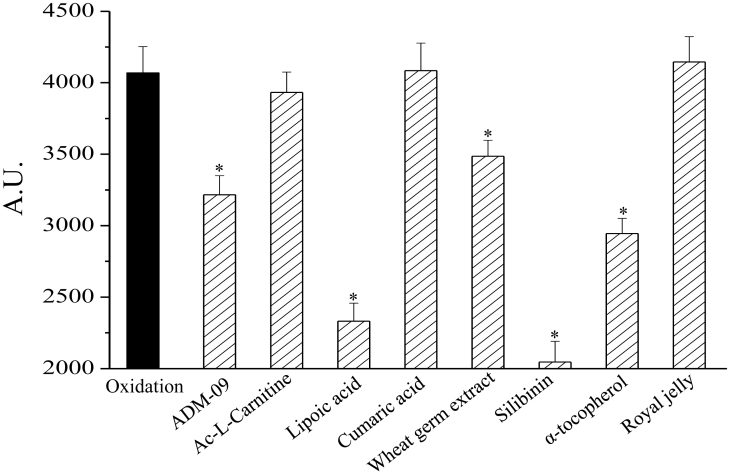
The antioxidant profile of ADM_09 was evaluated in vitro by measuring the oxidation of nitro blue tetrazolium (NBT) after 30 min, in the absence and in the presence of an increasing concentration of ADM_09 (Table S1, Supporting Information). The superoxide anion (O2−) generated by the hypoxanthine – xanthine oxidase system increased the oxidized NBT level from 100 (basal) to 4000 A.U. In the presence of ADM_09 in the reaction mixture, the oxidation of NBT was inhibited in a concentration-dependent manner. For example, 100 μM ADM_09 decreased the oxidized NBT from 4000 to 3200 A.U. (see Table S1), indicating a detectable antioxidative activity. However, comparing the efficacy of ADM_09 with other commonly employed antioxidants, including lipoic acid, it is easily appreciated that its antioxidative properties are only moderate and, notably, markedly less effective than the parent lipoic acid (Fig. 2). Apparently, coupling of lipoic acid with carnosine affected the antioxidative capability of the former residue, inducing a significant loss of activity.
Figure 2. Evaluation of the antioxidant properties of ADM_09 by the NBT assay.
O2− generated by hypoxanthine – xanthine oxidase reaction was used to oxidize NBT (Nitro Blue Tetrazolium). The oxidation was spectrophotometrically measured at 560 nm after 30 min, in the absence or in the presence of tested compounds (100 μM). Values are expressed as absorbance arbitrary units (A.U.). *P < 0.01 with respect to the reference value.
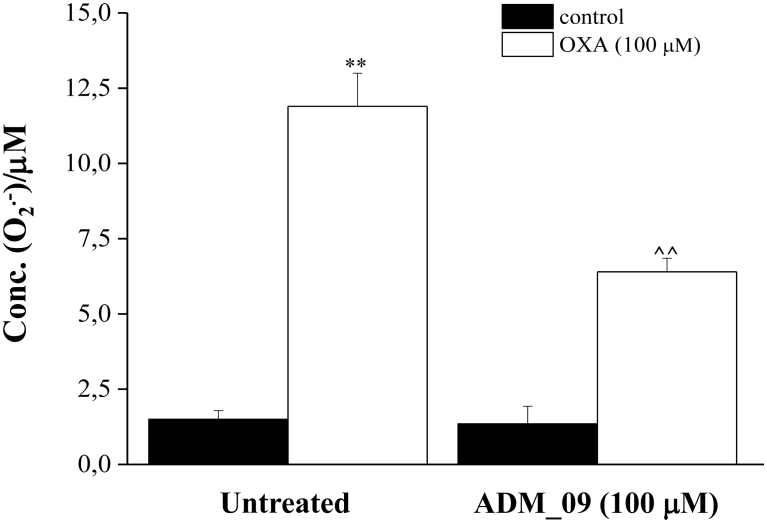
The antioxidative properties of ADM_09 were also tested in vivo in rat cortical astrocytes. Because glial cells exert a pivotal role in the development of neuropatic pain, astrocytes from primary cultures were used in the antioxidative tests, as these are directly related to normal tissues24. In primary cultures of astrocytes, the neurotoxic compound OXA (100 μM) induced a significant, SOD-inhibitable, superoxide anion increase after 4 h incubation, as evaluated by the cytochrome C assay (Fig. 3). Co-incubation with 100 μM ADM_09 inhibited the O2− formation by over 50% with respect to the untreated culture.
Figure 3. SOD-inhibitable superoxide anion levels in astrocyte cell lines.
Astrocytes (5 × 105 cells/well, 2 control wells and 2 wells pre-treated with ADM_09) were exposed to 100 μM OXA for 4 h. The effect of 100 μM ADM_09 co-incubation on SOD-inhibitable superoxide anion levels are depicted. The non-specific absorbance measured in the presence of SOD was subtracted to the total value. Values are expressed as mean ± s.e.m. of 3 experiments.**P < 0.01 vs control and ∧∧P < 0.01 vs OXA treatment.
Overall, these data indicate that ADM_09 possess antioxidative properties that may be beneficial for the OXA-induced oxidative stress, although showing lower activity compared to the parent lipoic acid.
Antihyperalgesic properties
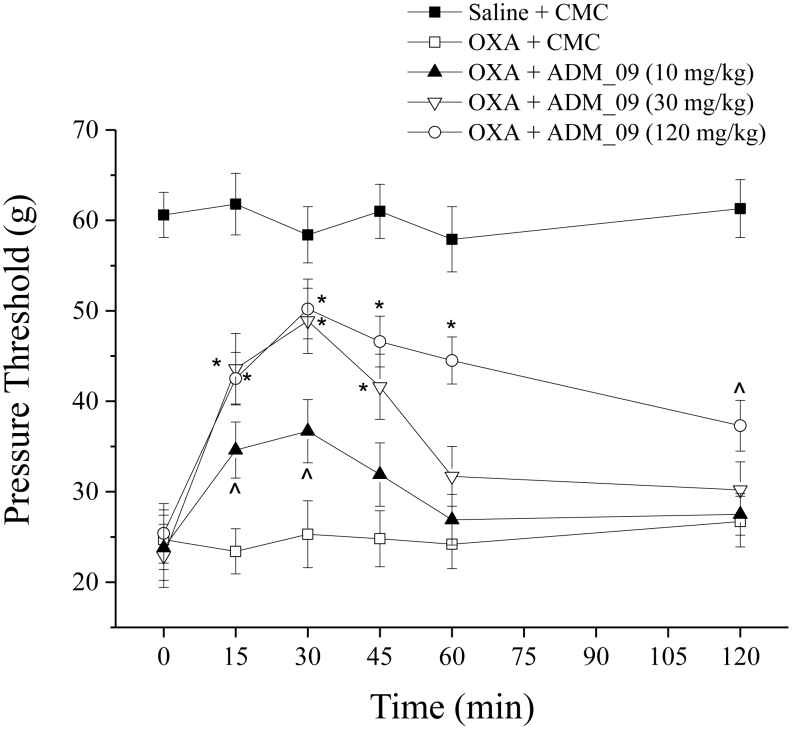
The efficacy of ADM_09 against NeP was tested in vivo by monitoring the response to a suprathreshold stimulation (hyperalgesia-related measure) in laboratory animals through the paw-pressure test25. OXA (2.4 mg/kg, i.p.) was administered daily to rats for 21 days according to the Cavaletti procedure25. To the treated rats, ADM_09 (10–120 mg/kg) was administered orally (p.o., vehicle: 1% CMC), following the effects at intervals from 15 to 120 min after administration. The recovery of pain threshold from OXA-induced hypersensitivity is reported in Figure 4 and Table S2 (see Supporting Information), in which it is clearly seen that ADM_09 reverts the decrease of pain threshold in a dose-dependent manner.
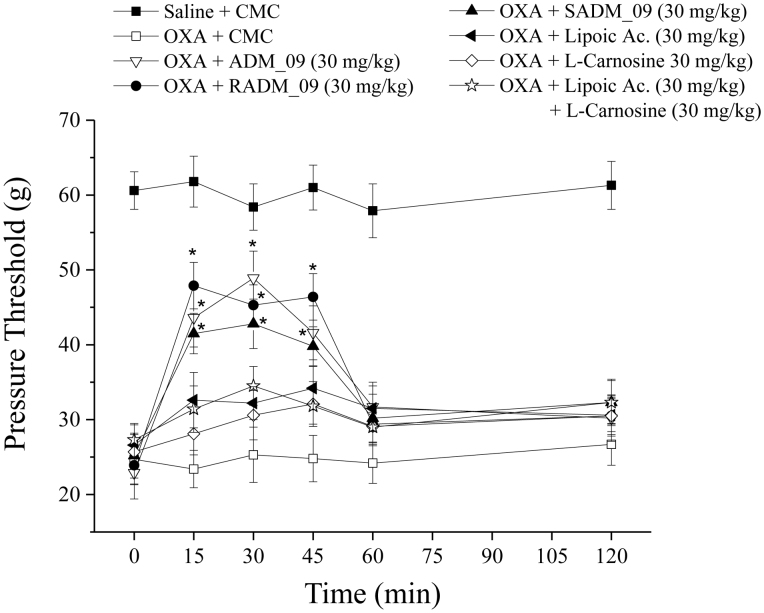
Figure 4. Pain threshold recovery from OXA-induced hypersensitivity.
Rats were treated daily intraperitoneally with OXA (2.4 mg/kg, i.p.). A time-dependent reduction in the mechanical paw-withdrawal threshold was produced. The response to the noxious mechanical stimulus was evaluated at day 21 by the paw-pressure test. At time 0, pure vehicle [1% carboxymethylcellulose (CMC)] or increasing doses of ADM_09 were administered per os (p.o.) and responses were recorded after 15, 30, 45, 60 and 120 min. Each value represents the mean from 12 rats per group, recorded in 2 different experimental set. ∧P < 0.05 and *P < 0.01 versus OXA + vehicle-treated rats.
The statistically significant effect could be observed for over 45 min after the administration, with a maximum efficacy at 30 min, whereas at the higher dose (120 mg/kg, p.o.) it persisted for up to 120 min after administration. At a dose of 30 mg/kg, over 70% of the normal pain threshold was recovered after 30 min, while no recovery at all was observed in the absence of ADM_09. At the same dose, the diastereomerically pure compounds RADM_09 and SADM_09 showed only slightly different activities (Fig. 5, Table S2), where RADM_09 appeared to be slightly more effective than SADM_09; however, the most relevant evidence was that both compounds were not significantly different from their mixture, validating the results from tests obtained with ADM_09.
Figure 5. Effect of ADM_09, RADM_09, and SADM_09 on pain threshold recovery from OXA-induced hypersensitivity.
Rats were treated daily with OXA (2.4 mg/kg, i.p.). A time-dependent reduction in the mechanical paw-withdrawal threshold was produced. The response to the noxious mechanical stimulus was evaluated at day 21 by the paw-pressure test. At time 0, pure vehicle [1% carboxymethylcellulose (CMC)] or individual compounds were administered per os at the dose of 30 mg/kg. (±) Lipoic acid, L-carnosine, and their 1:1 mixture were also administered as reference compounds. Each value is the mean from 12 rats per group, recorded in 2 different set. *P < 0.01 versus oxa + vehicle-treated rat.
It is noteworthy that neither lipoic acid nor carnosine at a dose of 30 mg/kg could revert OXA-induced NeP to more than a negligible extent; the same was true for their 1:1 combination at the same dose (Fig. 5, Table S2). This evidence clearly demonstrates that ADM_09 possess antihyperalgesic properties not shared by its constituting components; furthermore, the antioxidative tests described above suggest that the relief of NeP observed for treatment with ADM_09 cannot be ascribed to the antioxidative and ROS scavenging capabilities of its precursors.
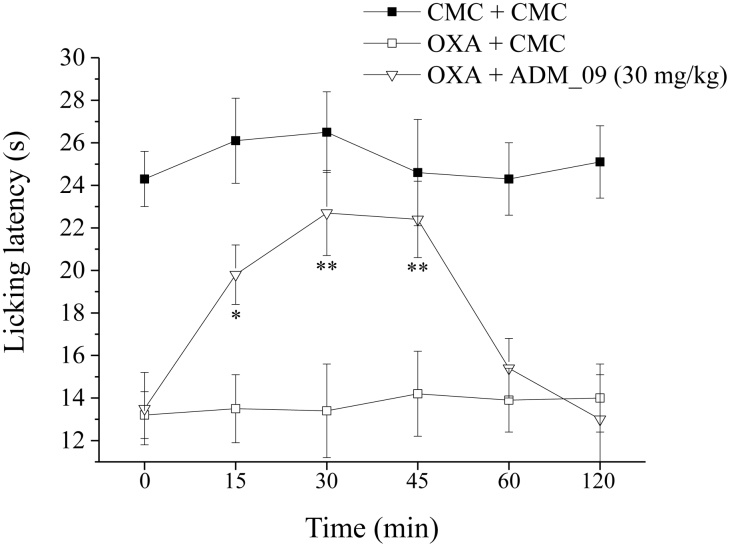
Cold allodynia, the hypersensitivity to cold temperatures, was described as a characteristic symptom in platin-treated patients26,27. In the present model of chemotherapy-induced neuropathy, on the day 21, oxaliplatin induced a decrease of pain threshold that could also be assessed by the cold plate test. After the administration per os to the treated rats of 30 mg/kg ADM_09, the hypersensitivity to cold temperatures was followed at intervals from 15 to 120 min. The resulting licking latency is reported in Figure 6 and Table S3 (Supporting Information), showing that a pain-reliever effect was already significant 15 min after treatment and persisted up to 60 min, with a maximum effect between 30 and 45 min. The results clearly show that the reduction in the licking latency to a non-noxious cold stimulus was effectively reverted by administration of ADM_09.
Figure 6. Effect of ADM_09 on OXA-induced cold allodynia.
Rats were treated daily intraperitoneally with oxaliplatin (2.4 mg/kg, i.p.). A time-dependent reduction in the licking latency to a cold non-noxious stimulus was produced. The response was evaluated at day 21 by the cold plate test. At time 0, pure vehicle [1% carboxymethylcellulose (CMC)] or 30 mg/kg ADM_09 were administered per os. Each value is the mean from 6 rats per group, recorded in 2 different set. *P < 0.05 and **P < 0.01 versus OXA + vehicle-treated rats.
It should be emphasized that, at 30 mg/kg, ADM_09 does not modify the normal behaviour in rats, as revealed by the lack of reduction of the motor coordination observable on rota-rod test (Table S4, Supporting Information). This property is of great pharmacological importance, considering that in lack of an effective treatment of NeP in general, and of chemotherapeutic-induced NeP in particular, the use of moderately effective drugs endowed with important negative side effects is usually unavoidable.
Toxicity
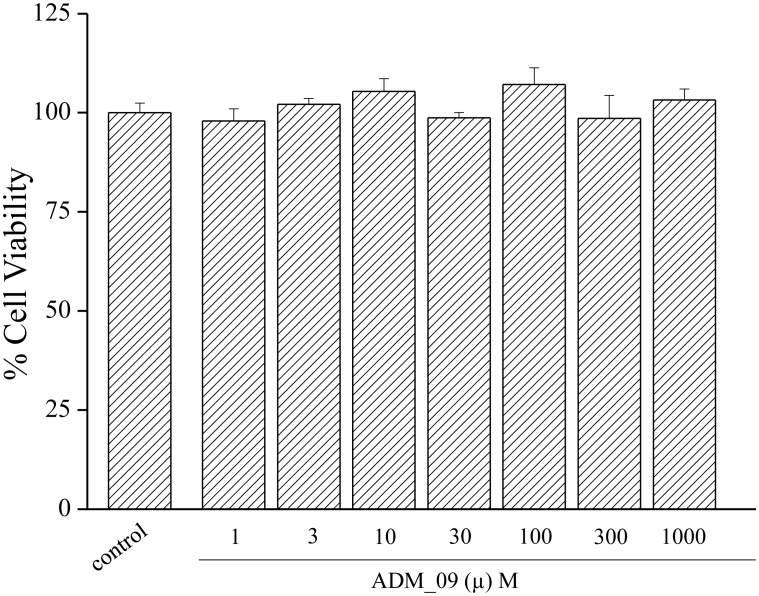
Toxicity is a crucial property for a promising antineuropathic compound. The toxicity of ADM_09 was tested in vitro on a primary culture of rat astrocytes. Cell viability was assessed as mitochondria functionality by MTT assay, incubating the cell cultures for 24 and 48 h in the presence of increasing amounts of ADM_09. The resulting cell viabilities, with respect to control in the absence of ADM_09, are reported in Figure 7 and Table S5 (Supporting Information) and clearly show the absence of any toxic effect on the astrocyte cultures.
Figure 7. Effect of ADM_09 on astrocyte cell viability.
Astrocytes (104 cells/well) were incubated with ADM_09 (1 – 1000 μM) for 24 and 48 hours. Viability was quantified by MTT assay. Absorbance was measured at 550 nm. Values are expressed in percentage of control absorbance as a mean ± s.e.m. of 6 experiments. Control values: 100.0 ± 2.4 (24 h); 100.0 ± 2.9 (48 h).
The reduction of risk of drug-induced cardiac arrhythmia is considered a major hurdle in the development of new drugs. For this reason the investigation of hERG channel blockage represents a fundamental step along the drug discovery process. The most common problem is the acquired long QT syndrome, that is caused by drugs that block the human ether-a-go-go related gene (hERG) protein28. Efforts to predict the cardiotoxicity of new drugs have been focused on assays testing hERG channel activities, since the blockage of hERG is considered an indicator of potential pro-arrhythmic risk.
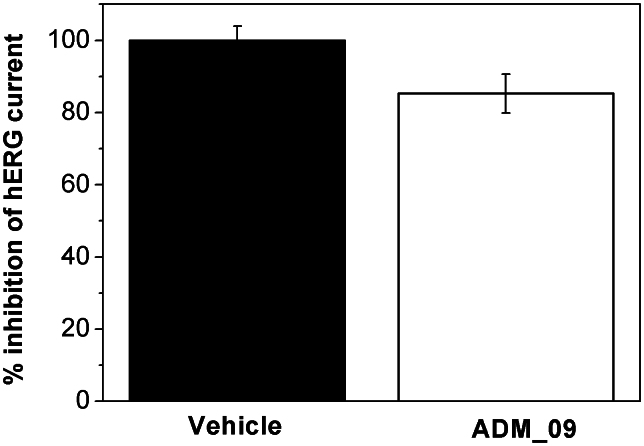
Cardiotoxicity was thus tested in vitro by recording whole-cell currents through HEK293 cells expressing hERG channels (Fig. 8). In the presence of 30 μM ADM_09, the measured outward current was reduced by 14.7 ± 5.4% with respect to the pure vehicle, indicating an IC50 > 30 μM. According to a general system for ranking the inhibitory potency toward the hERG channel29, compounds showing IC50 values larger than 10 μM are considered non-cardiotoxic. Thus, the hERG assay demonstrates that ADM_09 is largely above the toxicity threshold.
Figure 8. Effect of 30 μM ADM_09 on hERG channels expressed in HEK293 cells.
The outward current reduction was 14.7 ± 5.4% with respect to pure vehicle. hERG currents were elicited using a hyperpolarizing prepulse to −80 mV, followed by a voltage step from −60 to +40 mV for 500 ms, a step to −50 mV for 250 ms, and back to holding. The variation of the K+ current at +40 mV is reported. Each column represents the mean ± SEM of n > 5 cells.
ADM_09 is a TRPA1 antagonist
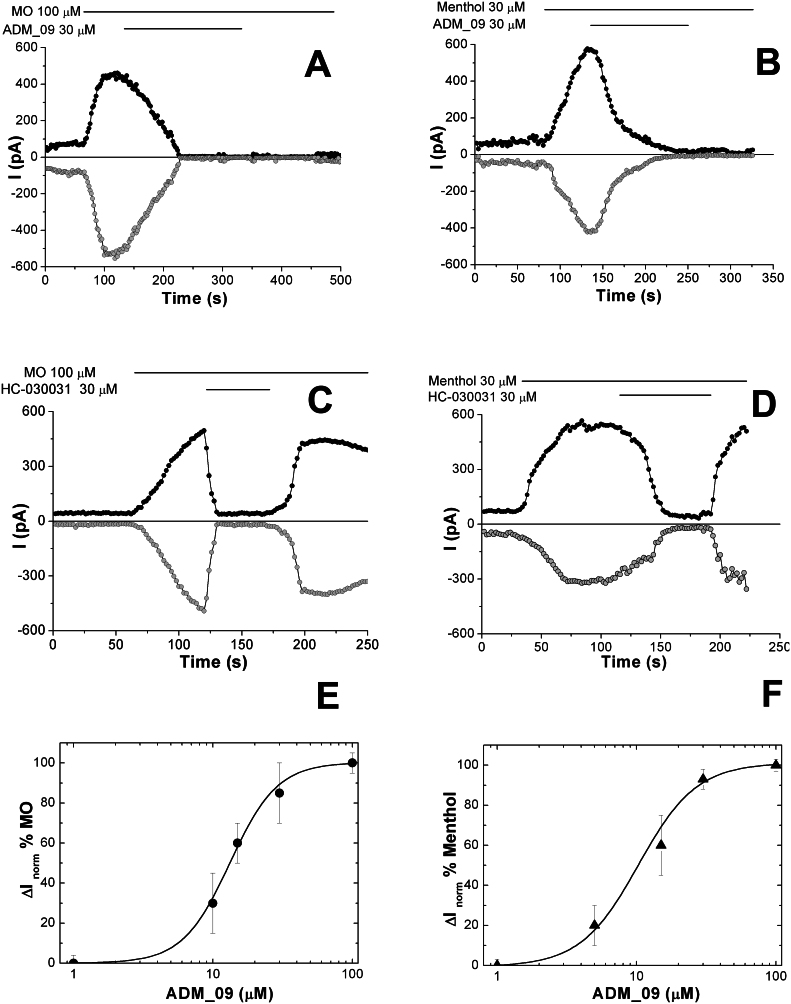
Because of the putative role of TRPA1 in OXA-induced hyperalgesia and cold allodynia, our attention was focused on this sensor channel. The TRPA1 channel is activated by several mechanisms and has been actively investigated as a target to control NeP30,31. A chemical activation mechanism of TRPA1 as a nociceptive sensor involves the interaction of its cysteine residues with reactive compounds, which causes the opening of the channel. Because the ADM_09 disulphide bridge may react with the thiol groups of TRPA1 cysteine residues, to shed light on the origin of the antihyperalgesic properties of ADM_09, the interaction with TRPA1 was investigated through whole-cell patch-clamp recordings. Chinese Hamster ovary (CHO) cells, transfected to overexpress mTRPA1 from mice, were treated by perfusion with OXA, with ADM_09, and with well-known activators and blockers of TRPA1 as reference competitors. Both, covalent (allyl isothiocyanate from mustard oil – MO) and noncovalent (menthol) activators were selected for testing, while HC-030031 was employed as the most widely used noncovalent blocker. The above compounds were independently fed to the cell culture at discrete intervals, recording the resulting whole-cell current measured for membrane potential ramps from –100 to + 100 mV. In the case of TRPA1, it has been shown that the observed current is mainly due to a flux of Na+ and Ca2+ ions through the channel, flowing inward for negative potentials (i.e., under physiological conditions) and outward for positive potentials32. Because the TRPA1 channel is voltage-dependent31, currents were sampled at membrane potentials of +50 and –50 mV in all the experiments. The time courses obtained for the whole-cell current and the corresponding voltage ramps are reported in Figure 9 and Figure S1 (Supporting Information), respectively, in which the compounds perfused, their concentration, and the perfusion time intervals are indicated.
Figure 9. Representative time courses of whole-cell currents through mTRPA1-expressing CHO, measured at membrane potential of +50 mV (black curve) and −50 mV (grey curve) during administration of A) MO and ADM_09; B) Menthol and ADM_09; C) MO and HC030031; D) Menthol and HC030031 at the indicated concentrations and time intervals.
E) Dose-response curve for the inhibition of 100 μM MO-induced currents by ADM_09. The corresponding IC50 value is 13.2 ± 0.5 μM. F) Dose-response curve for the inhibition of 100 μM menthol-induced currents by ADM_09. The corresponding IC50 value is 10.3 ± 1.7 μM.
When MO is administered (Fig. 9A), a current increase is observed for both positive and negative potentials, which is driven to zero by perfusion of ADM_09. Similarly, a current is recorded when menthol is administered, which is damped when ADM_09 is added (Fig. 9B). Clearly, ADM_09 behaves as an antagonist of the channel, blocking the stimulated currents caused by the activators administered. In this phenomenology some features are noteworthy: (a) current traces are symmetrical with respect to positive and negative membrane potentials, showing that the channel is gated the same way in both, the inward and the outward directions; (b) current is fully depleted by ADM_09, whereas it is non-zero before activation; (c) most importantly, the channel does not re-activate if the activator is fed after the perfusion of ADM_09 has been suspended. Apparently, the blocking effect of ADM_09 persists beyond the time of administration, a feature that is not observed when HC-030031 is the antagonist employed (the actual persistence of the blocking effect has been observed for at least 10 min after the perfusion of ADM_09 has been suspended). Indeed, administration of HC-030031 after activation with MO (Fig. 9C) or menthol (Fig. 9D) blocks the channel for the time of perfusion, but re-activation is immediately observed if the administration is suspended while the activator keeps being fed. Note that the initial current is restored by HC-030031, rather than fully depleted, as with ADM_09. In Figure 9E and 9F, the dose-response curves for the inhibition of MO-induced and menthol-induced currents by ADM_09 are reported, respectively; the corresponding IC50 values are 13.2 ± 0.5 μM and 10.3 ± 1.7 μM, respectively, showing with excellent agreement a remarkably effective blocking activity of ADM_09 toward the TRPA1 channel.
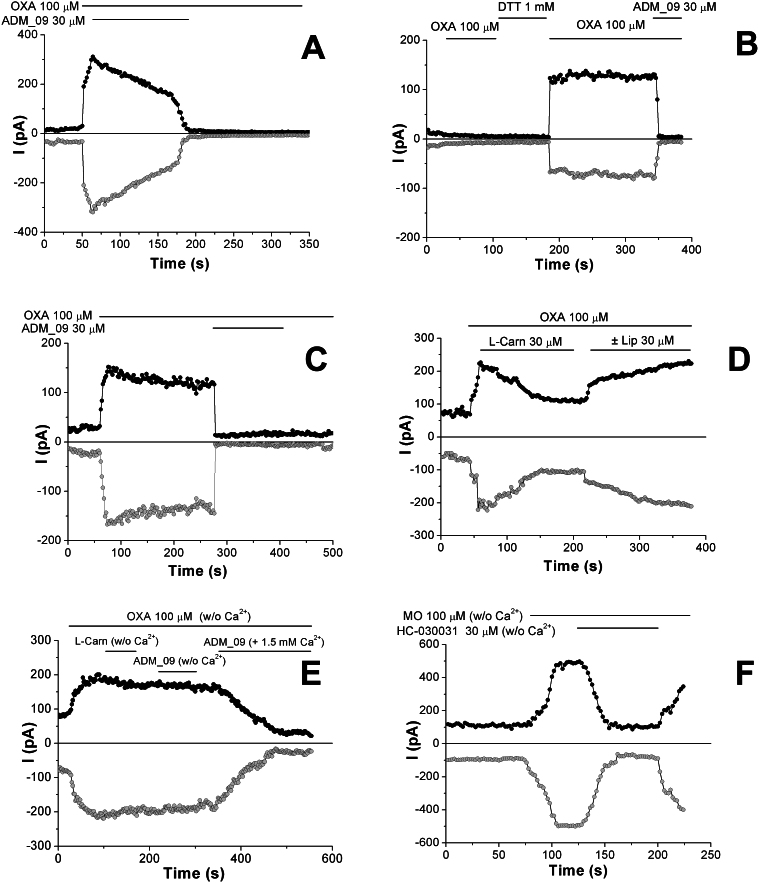
To relate the TRPA1 blocking activity of ADM_09 with the observed antihypralgesic effects, whole-cell currents caused by the administration of OXA were recorded. The corresponding time courses and voltage ramps are reported in Figure 10 and S2 (Supporting Information), respectively.
Figure 10. Representative time courses of whole-cell currents through mTRPA1-expressing CHO, measured at membrane potential of +50 mV (black curve) and −50 mV (grey curve) during perfusion, at the indicated concentrations and time intervals, of A) OXA and ADM_09; B) OXA, DTT, OXA, and ADM_09, on cell cultures preincubated with 30 μM ADM_09 for 10 min at r.t.; C) OXA and ADM_09 on human hTRPA1-expressing CHO; D) OXA, L-carnosine, and (±) lipoic acid; E) Ca2+-free solutions of OXA, L-carnosine, and ADM_09 and, eventually, a Ca2+-containing solution of ADM_09; F) Ca2+-free solutions of MO and HC-030031.
In Figure 10A, a steep rise of the current is observed after perfusion of OXA, which is driven to zero in 100 s ca by the administration of ADM_09. Clearly, OXA activates TRPA1, whereas ADM_09 exerts a channel blocking effect on the OXA-induced currents (a direct chemical reaction of ADM_09 with OXA can be ruled out on the basis of 1H NMR spectra, showing the absence of any spectral variation after 24 h when mixing the two compounds in water at room temperature). Consistently, as observed with MO and menthol, the channel is not re-activated when perfusion of ADM_09 is suspended, even under continuing administration of OXA; this evidence confirms that ADM_09 acts as an antagonist steadily reverting the OXA-induced noxious effects. In agreement with this evidence, preincubation of TRPA1-expressing cells with ADM_09 renders the channel insensitive to OXA administration (Fig. 10B). Interestingly, TRPA1 activation is restored when the blocked channel is treated with dithiothreitol (DTT), a standard reducing agent of disulphide bridges; the restored current is again turned off by ADM_09 administration. This sequence of events demonstrates that ADM_09-induced blockage is reversible, and can be reverted by reducing agents.
It is known that human and rodent TRPA1 homologues display striking differences in their activities. Often, compounds showing antagonistic effect with human TRPA1 can behave as agonists or show no activity in rat and mouse31,33. To validate the results obtained on mTRPA1, wild-type human hTRPA1-expressing CHO were tested by treatment with OXA. The current traces and the voltage ramps recorded as whole-cell currents, reported in Figure 10C and S2C, respectively, demonstrate that the behavior of ADM_09 toward the two TRPA1 homologues is strictly analogous, showing channel opening by OXA and blockage by ADM_09 that persists under continuing perfusion of OXA.
Because the activity-restoring effect of DTT suggests that the disulphide bridge of lipoic acid may be involved in the blocking activity of ADM_09, independent administration of equimolar amounts of L-carnosine and (±) α−lipoic acid were performed while continuously feeding OXA. The current traces reported in Figure 10D and the voltage ramps depicted in Figure S2D clearly show that partial blocking is induced by carnosine, whereas lipoic acid does not exert any blocking effect on the OXA-induced activation. The results suggest that, although the antioxidative properties of lipoic acid may play some role in the function of ADM_09, the blocking effect appears rather to be related to the carnosine moiety. Yet, the blocking activity of ADM_09 toward TRPA1 is markedly stronger than that of both the constituting building blocks, a feature in agreement with the antihyperalgesic effects observed in vivo; in addition, the persistent blocking effect after administration of ADM_09 is absent in the carnosine-induced blockage. Quite surprisingly, when treating OXA-activated TRPA1 with L-carnosine or with ADM_09 in the absence of extracellular Ca2+, no blocking activity could be detected from the current recordings (Figs. 10E and S2E), unexpectedly showing that both compounds are calcium-dependent blockers. Accordingly, the blocking activity is restored when feeding a Ca2+-containing solution of ADM_09 (Fig. 10E). Thus, the blocking properties of ADM_09 are modulated by the Ca2+ concentration. In contrast, as a control reference, the described behavior is not evoked by HC-030031 on MO-activated TRPA1 (Figs. 10F and S2F), which is insensitive to the absence of calcium ions.
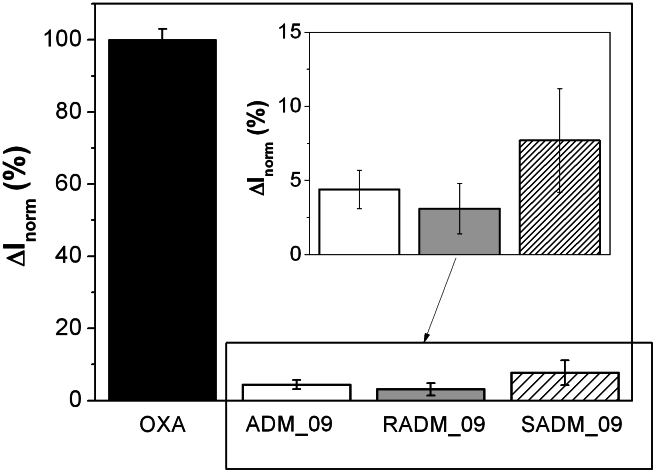
Finally, the different antihyperalgesic activity observed in vivo for the two diastereomeric structures of ADM_09, featuring the R diastereomer slightly more effective than the S isomer, was checked by recording the whole-cell currents through mTRPA1 after activation by OXA and blockage with RADM_09 and SADM_09. The results reported in Figure 11 as normalized currents show that, with respect to OXA, the observed residual current is 3% for RADM_09 and 8% for SADM_09, with an intermediate value of over 4% for their mixture present in ADM_09. It should be appreciated that current data obtained in vitro from the TRPA1 channel closely match the in vivo antihyperalgesic results from the paw-pressure and the cold plate tests, strongly supporting a direct connection between the ability of ADM_09 to revert NeP and its TRPA1 blocking activity.
Figure 11. Pooled-data of whole-cell currents through mTRPA1-CHO transfected cells measured at membrane potential of +50 mV, activated by oxaliplatin 100 μM (OXA, black) and blocked by ADM_09 (white), RADM_09 (grey) and SADM_09 (hatched) 30 μM each.
Each column is the mean ± SEM of n > 5 cells. Values are normalized to OXA-activated channel current. Whole-cell currents were elicited by 400 ms voltage ramps from -100 to +100 mV. The residual TRPA1 current was 4.4 ± 1.3% for ADM_09; 3.1 ± 1.7% for RADM_09; and 7.7 ± 3.5% for SADM_09, respectively.
Discussion
Gathering all the results presented, a rationale for the blocking activity of ADM_09 can be tentatively puzzled out. TRPA1 is a Ca2+ permeable non-selective cation channel; its activity is not only calcium-regulated, but also characterized by a large fraction of the inward current being carried by Ca2+. Stimulation by activators, such as MO, induces an increase in the fractional Ca2+ current, suggesting that the pore of TRPA1 is dynamic and supports a surprisingly large Ca2+ influx31. In addition, Ca2+ binds to TRPA1, and the putative Ca2+ -binding domain is constituted by acidic residues located in the pore near the C- terminus of the channel. TRPA1 is particularly rich in cysteine residues, that constitute the nucleophilic sites of disulphide bond formation with thiol-reacting compounds, which activate the nociceptive response30.
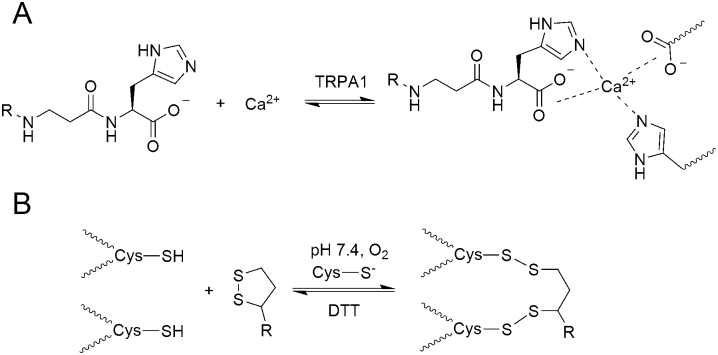
The patch-clamp experiments described in this work have shown that carnosine and the carnosine moiety of ADM_09 are the structural units responsible for blocking the activated TRPA1, although with different efficacy, and that both require Ca2+ ions to exert blocking activity. This evidence is in agreement with the calcium-binding properties of carnosine: under physiological conditions, carnosine forms monomeric (through carboxylate and carbonyl oxygens) and dimeric (through carboxylate, carbonyl oxygens, and imidazole nitrogens) complexes with Ca2+ 34. It is therefore predictable that carnosine may form a ternary complex with Ca2+ and TRPA1, in which carboxylate and imidazolic groups from the channel protein would replace one of the two carnosine molecules in the complex, as depicted in Figure 12A. This may explain the calcium-mediated binding of carnosine to the TRPA1 pore; yet, the moderate blocking activity may be ascribed to the intrinsic lability of the complex, as desumed from the moderate affinity reported for the carnosine Ca2+ complex34. In contrast, a markedly stronger blocking effect is exerted by ADM_09, and this enhancement can be explained on the basis of the well-established thiol-disulphide chemistry (Fig. 12B).
Figure 12.
(A) Interaction of carnosine residues with Ca2+ ions and carboxylate and imidazole groups from TRPA1, leading to a ternary complex. (B) Thiolate-catalyzed disulphide bridge formation between TRPA1 cysteines and lipoic acid disulphide. The reaction is reversed by reduction with dithiotreitol.
At physiological pH, thiol groups are converted into disulphides by atmospheric oxygen. This thermodynamically-favored process occurs concomitantly to the thiolate-catalyzed thiol/disulphide equilibration, which scrambles the cysteine thiol groups of the protein with the sulphur atoms of the lipoic acid disulphide groups, thereby anchoring ADM_09 to the channel through disulphide bridges. In this process, the catalytic thiolate anion required for the equilibration is ensured by the imidazolic groups of hystidines. Through this pathway, the calcium-binding carnosine moiety is covalently bound to the channel through the lipoic acid residue, and can therefore exert a persistent activity through a synergically stabilized binding to TRPA1. Consistently, ADM_09 can be “washed away” by DTT, which reverts the disulphide bridging to the channel by restoring the proteic cysteine thiol groups and the disulphide moiety of lipoic acid. The activation/blocking cycle is depicted in the scheme of Figure 13. Note that even in the presence of Ca2+, lipoic acid alone is incapable of blocking TRPA1, whereas in the absence of Ca2+, ADM_09 is devoid of any blocking activity as well, although disulphide anchoring is most likely occurring in both cases. Thus, ADM_09 is a dual-binding calcium-dependent blocker of TRPA1 in that the Ca2+-binding carnosine and the lipoic acid disulphide are both essential requirements for activity.
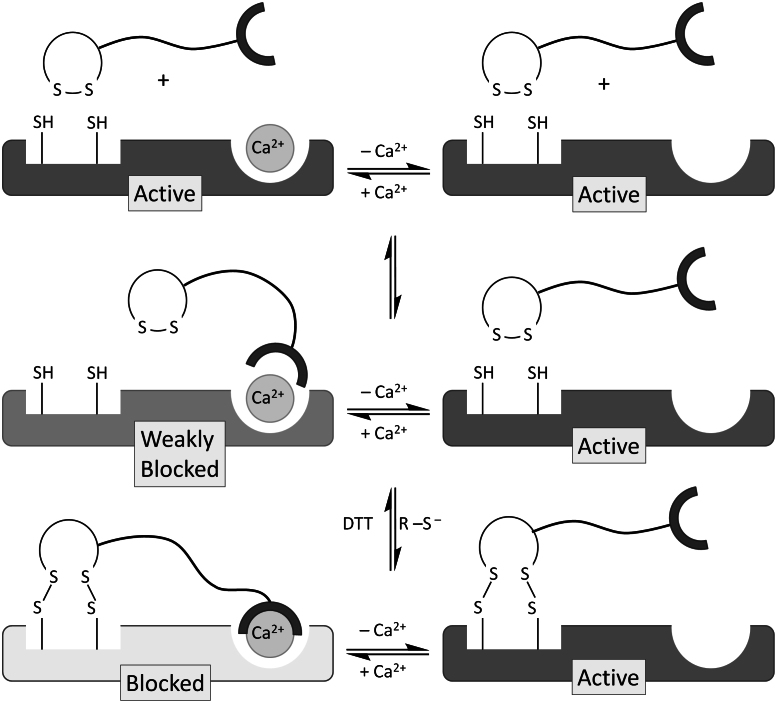
Figure 13. Schematic representation of the interaction of ADM_09 with TRPA1.
Binding of the carnosine residue of ADM_09 to TRPA1 is mediated by Ca2+ through the formation of a ternary complex. Partial blocking is induced by the moderate affinity of carnosine for Ca2+ ions. Covalent anchoring of the lipoic acid residue through disulphide bridges synergically stabilizes the ADM_09 – TRPA1 adduct, evoking a persistent deactivation of the channel and blockage of the measured current. The process is reverted by reduction with DTT, which breaks disulphide bridges restoring cysteine thiol and lipoic acid disulphide groups. Note that in the absence of Ca2+ ions, TRPA1 is not deactivated by ADM_09, although disulphide anchoring is most likely occurring.
The actual blocking mechanism by which the bound ADM_09 can prevent the current from flowing through the channel has yet to be elucidated, although allosteric modifications of the pore induced by the significant size of the bound structure may represent a reasonable working hypothesis. Nevertheless, a phenomenological aspect of the mode of action we propose deserves a comment. It is generally accepted that disulphide-bond-forming reactive compounds cause activation of the TRPA1 channel by covalent modification of reactive cysteines, whereas channel blocking is induced by the reducing agent DTT. In our results, the opposite seems to occur, whereby disulphide bond formation causes deactivation of TRPA1, whereas DTT restores the native form of the channel that is activated by OXA. This apparent discrepancy can be rationalized considering that the actual blocking effect is due to the calcium-mediated binding of carnosine, where the disulphide bridges merely exert the function of anchoring ADM_09 to the proteic backbone. It should also be considered that, most likely, the cysteines involved in the anchoring of ADM_09 are different from those involved in the activation of the channel. Indeed, when anchoring lipoic acid, or ADM_09 in the absence of Ca2+, on TRPA1 activated with MO, no blocking effect is observed, indicating that the two disulphide-forming reactants do not interfere with each other.
Concluding, in the present work we have shown that ADM_09, obtained by coupling L-carnosine with α-lipoic acid, can effectively revert neuropathic pain evoked by chemotherapeutic agents, such as oxaliplatin, without eliciting the most common negative side-effects observed in pain treatment. While exhibiting reduced antioxidative properties with respect to the parent lipoic acid, ADM_09 showed a marked efficacy in neuropathic pain control in vivo, which was lacking in both the parent constituents. Most importantly, ADM_09 did not modify the normal behavior in rat, and did not show any toxicity toward astrocyte cell viability, nor any relevant cardiotoxicity, as revealed by the human ERG channel test. Patch-clamp experiments demonstrated that ADM_09, in contrast to both L-carnosine and α-lipoic acid, is a TRPA1 antagonist capable of blocking currents evoked by several covalent and noncovalent activators, including oxaliplatin, thus reverting the neuropathic response. A rationale for the observed blocking activity has been proposed, according to which the persistent antagonistic effect of ADM_09 toward TRPA1 is caused by a synergic combination of a deactivating calcium-mediated binding of the carnosine residue and of a covalent anchoring of the lipoic acid residue to the cysteines of the channel protein. It should be emphasized that the antagonistic effects of ADM_09 on the human TRPA1 variant are strictly analogous to those observed in the experiments with the rodent homologues. Although the actual mechanism of action has yet to be established, the findings reported in this work open the way to the development of a new generation of compounds for neuropathic pain control.
Methods
Cell Viability Assay
Cell viability was evaluated by the reduction of 3-(4,5-dimethylthiozol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) as an index of mitochondrial compartment functionality. Primary astrocytes were plated into 96 well cell culture plates, grown until confluence and treated for 24 and 48 hours with different concentrations of ADM_09 in DMEM. After extensive washing, 1 mg/mL MTT was added into each well and incubated for 2 hours at 37°C. After washing, the formazan crystals were dissolved in 100 μL dimethyl sulfoxide. The absorbance was measured at 580 nm. Experiments were performed in quadruplicate on at least 3 different cell batches.
Animals
Male Sprague-Dawley rats (Harlan, Varese, Italy) were used, weighing approximately 200–250 g at the beginning of the experimental procedure. The animals were housed in CeSAL (Centro Stabulazione Animali da Laboratorio, University of Florence) and used at least one week after their arrival. Four rats were housed per cage (size 26 × 41 cm); the animals were fed with standard laboratory diet and tap water ad libitum, and kept at 23 ± 1°C with a 12 h light/dark cycle, light at 7 a.m. All animal manipulations were carried out according to the European Community guidelines for animal care (DL 116/92, application of the European Communities Council Directive of 24 November 1986 (86/609/EEC). Formal approval to conduct the experiments described was obtained from the Animal Subjects Review Board of the University of Florence. The ethical policy of the University of Florence complies with the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health (NIH Publication No. 85–23, revised 1996; University of Florence assurance number: A5278-01). All efforts were made to minimize animal suffering and to reduce the number of animals used.
CHO cell culture
We used a tetracycline-regulated system for inducible expression of mouse TRPA1 in CHO (Chinese-Hamster Ovary) cells as previously reported15. To induce expression of TRPA1, 1 μg/ml tetracycline was added to the culture medium, and cells were used 2–3 h after induction.
Author Contributions
C.N., C.G., M.R.M. and S.R. designed research; R.G., E.D., L.D.C.M., S.S., M.N., G.G., G.l.M., B.R. and O.F. performed research; C.N., C.G., R.G. and S.R. analyzed data and C.N. and S.R. wrote the paper.
Supplementary Material
A TRPA1 antagonist reverts oxaliplatin-induced neuropathic pain
Acknowledgments
We are grateful to prof. Pierangelo Geppetti (University of Florence) for providing mouse TRPA1-CHO stably transfected cells and HC030031, to prof. David Julius (UCSF, CA USA) for providing human TRPA1 wild-type cDNA and to prof. Annarosa Arcangeli (University of Florence) for the kind gift of HEK 293 cells stably expressing hERG channel. This work was supported by the Ministero dell'Istruzione, dell'Università e della Ricerca, AIRC and the Ente Cassa di Risparmio di Firenze.
References
- Sherrington C. S. Observation on the scratch-reflex in the spinal dog. J. Physiol. 34, 1–50 (1905). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf C. J. & Mannion R. J. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet 353, 1959–1964 (1999). [DOI] [PubMed] [Google Scholar]
- Gilron I., Watson C. P., Cahill C. M. & Moulin D. E. Neuropathic pain: a practical guide for the clinician. Can. Med. Ass. J. 175, 265–275 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R. Mechanisms of disease: neuropathic pain--a clinical perspective. Nat. Clin. Pract. Neurol. 2, 95–106 (2006). [DOI] [PubMed] [Google Scholar]
- White F. A., Jung H. & Miller R. J. Chemokines and the pathophysiology of neuropatic pain. Proc. Natl. Acad. Sci. USA 104, 20151–20158 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C. P. The treatment of neuropathic pain: antidepressant and opioids. N. Engl. J. Med. 343, 1563–5 (2000). [DOI] [PubMed] [Google Scholar]
- Hempenstall K. et al. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2, 628–644 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy J. D. Neuropatic pain molecular complexity underlines continuing unmet medical need. J. Med. Chem. 50, 2547–2556 (2007). [DOI] [PubMed] [Google Scholar]
- Grothey A. Clinical management of oxaliplatin-associated neurotoxicity. Clin. Colorectal. Cancer 1, S38–46 (2005). [DOI] [PubMed] [Google Scholar]
- Cersosimo R. J. Oxaliplatin-associated neuropathy: a review. Ann. Pharmacother. 39, 128–35 (2004). [DOI] [PubMed] [Google Scholar]
- Andrade E. L., Meotti F. C. & Calixto J. B. TRPA1 antagonists and potential drugs. Pharmacology & Therapeutics 133, 189–204 (2012). [DOI] [PubMed] [Google Scholar]
- Kiernan M. C. The pain with platinum: oxaliplatin and neuropathy. Eur. J. Cancer 43, 2631–2633 (2007). [DOI] [PubMed] [Google Scholar]
- Argyriou A. A., Bruna J., Marmiroli P. & Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Crit. Rev. Oncol. Hematol. 82, 51–77 (2012). [DOI] [PubMed] [Google Scholar]
- Gauchan P., Andoh T., Kato A. & Kuraishi Y. Involvement of increased expression of transient receptor potential melastatin 8 in oxaliplatin-induced cold allodynia in mice. Neurosci. Lett. 458, 93–95 (2009). [DOI] [PubMed] [Google Scholar]
- Nassini R. et al. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain 152, 1621–1631 (2011). [DOI] [PubMed] [Google Scholar]
- Descoeur J. et al. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol. Med. 3, 266–278 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. W. et al. Broad neuroprotective profile of nicotinamide in different mouse models of MPTP-induced parkinsonism. Eur. J. Neurosci. 28, 610–617 (2008). [DOI] [PubMed] [Google Scholar]
- Nativi C., Ghelardini C. & la Marca G. Compounds with both analgesic and anti-hyperalgesic efficacy. PCT/IB2011/050018.
- Saada M. C. et al. Carbonic anhydrase activators: glod nanoparticles coated with derivatized histamine, histidine, and carnosine show enhanced activatory effects on several mammalian isoforms. J. Med. Chem. 54, 1170–1177 (2011). [DOI] [PubMed] [Google Scholar]
- Parcker L., Witt E. H. & Tritschler H. J. Alpha-lipoic acid as a biological antioxidant. Free Radical Biol. Med. 19, 227–250 (1995). [DOI] [PubMed] [Google Scholar]
- Joseph E. K., Chen X., Bogen O. & Levine J. D. Oxaliplatin on IB4-positive nociceptors to induce an oxidative stress-dependent acute painful peripheral neuropathy. J. Pain 9, 463–472 (2008). [DOI] [PubMed] [Google Scholar]
- Hipkiss A. R. Carnosine and its possible roles in nutrition and health. Adv. Food Nutr. Res. 57, 87–154 (2009). [DOI] [PubMed] [Google Scholar]
- Lipinski C. A. et al. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development setting. Adv. Drug Delivery Reviews 23, 3–25 (1997). [DOI] [PubMed] [Google Scholar]
- Scholz J. & Woolf C. J. The neuropatic pain triad: neurons, immune cells and glia. Nat. Neurosci. 10, 1361–1368 (2007). [DOI] [PubMed] [Google Scholar]
- Cavalletti G. et al. Effects of different schedules of oxaliplatin treatment on the peripheral nervous system of the rat. Eur. J. Cancer 37, 2457–2463 (2001). [DOI] [PubMed] [Google Scholar]
- Kannarkat G., Lasher E. E. & Schiff D. Neurologic complications of chemotherapy agents. Current Opinion in Neurology 20, 719–725 (2007). [DOI] [PubMed] [Google Scholar]
- Wolf S. et al. Chemotherapy induced peripheral neuropathy: prevention and treatment strategies. Eur. J. Cancer 44, 1507–1515 (2008). [DOI] [PubMed] [Google Scholar]
- Mitcheson J. S. et al. A structural basis for drug-induced long QT syndrome. Proc. Natl. Acad. Sci USA 97, 12329–12333 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche O. et al. A Virtual Screening Method for Prediction of the hERG Potassium Channel Liability of Compound Libraries. ChemBioChem 3, 455–9 (2002). [DOI] [PubMed] [Google Scholar]
- Nilius B., Appendino G. & Owsianik G. The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflugers Arch –Eur J Physiol 464, 425–458 (2012). [DOI] [PubMed] [Google Scholar]
- Karashima Y. et al. TRPA1 acts as a cold sensor in vitro and in vivo. Proc. Natl. Acad. Sci USA 106, 1273–1278 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima Y. et al. Agonist-induced changes in Ca2+ permeation through the nociceptor cation channel TRPA1. Biophysical J. 98, 773–783 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt P. M. A TRP(i)P in the air. Nature Chemical Biology 7, 661–663 (2011). [DOI] [PubMed] [Google Scholar]
- Gaggelli E. & Valensin G. 1H and 13C NMR Relaxation Investigation of the calcium complex of beta-alanyl-L-histidine (carnosine) in aqueous solution. J. Chem. Perkin Trans. 2, 401–406 (1990). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A TRPA1 antagonist reverts oxaliplatin-induced neuropathic pain