Fig. 1.
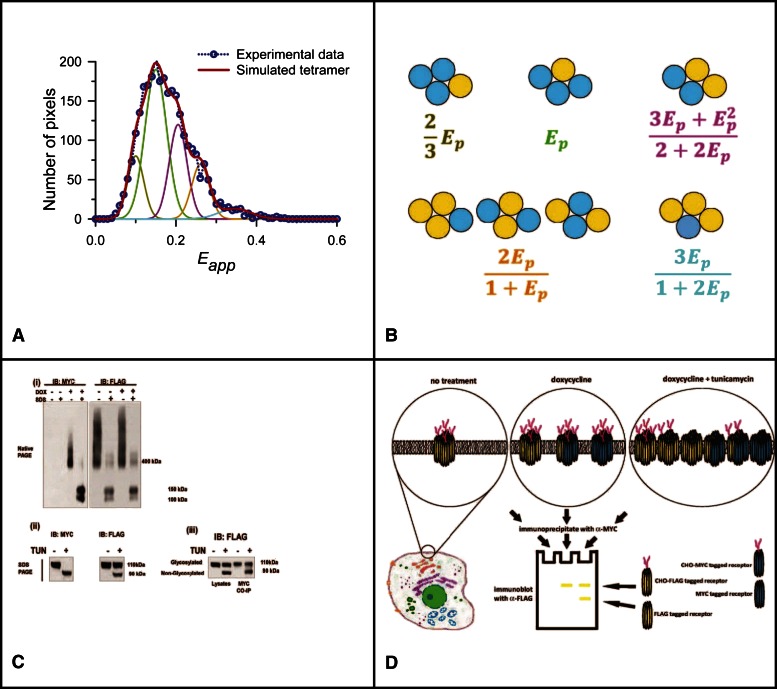
Spectrally resolved FRET and coimmunoprecipitation studies define the complex organization and dynamics of the muscarinic M3 receptor quaternary complex at the surface of cells. Spectrally resolved FRET was used to investigate the organization of the muscarinic M3 receptor at the surface of Flp-In T-REx 293 cells that constitutively express a form of the muscarinic M3 receptor that contains an N-terminal FLAG epitope tag and with the fluorescent protein citrine linked-in frame to the C terminus (the energy acceptor) and harbor at the inducible Flp-In T-REx locus, a variant of this receptor containing an N-terminal MYC epitope and C-terminal cerulean fluorescent protein (the energy donor). Expression from the Flp-In T-REx locus is controlled by the addition of various concentrations of doxycycline (DOX) (A). A broad range of FRET efficiencies were measured at the surface of cells induced to express varying amounts of the energy donor. This is not consistent with the receptor existing simply as a dimer because this scenario would be anticipated to result in a single FRET efficiency peak. Analysis of the experimental data based on the receptor existing, at least partially, as a rhombic tetramer with individual species containing varying numbers of acceptor (yellow) and donor (blue) species (B) showed that the experimentally derived and simulated results were highly similar (A). (C) Lysates from cells as described earlier were resolved by either Native-PAGE (i) or SDS-PAGE (ii–iii). Immunoblots of Native-PAGE resolved samples confirmed doxycycline-induced expression of the MYC-tagged protein and both constitutive and maintained expression of the FLAG-tagged form, whereas addition of SDS to samples prior to addition to the native gel resulted in production of two, potentially differentially N-glycosylated, variant monomer forms of each (i). Treatment of cells with PNGaseF confirmed this (not shown). Maintenance of the cells in the presence of the de novo N-glycosylation inhibitor tunicamycin (TUN) during the period of induction of the MYC-tagged energy donor resulted in all cell surface anti-MYC reactivity lacking carbohydrate (90 kDa), whereas only the proportion of the FLAG-tagged energy acceptor synthesized during this period lacked N-linked glycosylation (ii). Anti-MYC immunoprecipitates performed on lysates from cells treated with both doxycycline and tunicamycin resulted in the coimmunoprecipitation of both N-glycosylated (110 kDa) and nonglycosylated (90 kDa) FLAG-tagged energy acceptor (iii). This must reflect the presence of FLAG-tagged proteins both made before and after addition of tunicamycin in complex with the MYC-tagged variant, and therefore dynamic interactions between the forms. Results are adapted from Patowary et al. (2013). (D) An illustration representation of the experiments illustrated in (C), based on a tetrameric organization of the M3 receptor. For “no treatment,” i.e., in the absence of doxycycline, only the energy acceptor species (yellow) is present and all copies are N-glycosylated. Following addition of doxycycline, a mixture of energy donors (blue) and acceptors are present, and all are N-glycosylated and exist as a mixture of homo- and heterotetramers. With cotreatment of the cells with doxycycline and tunicamycin, a more complex pattern is predicted in which all energy donors lack N-glycosylation, whereas, based upon their time of synthesis, the energy acceptor species may be either N-glycosylated or nonglycosylated. As shown in (Ciii), immunoprecipitation of the MYC-labeled energy donor results in the coimmunoprecipitation of both N-glycosylated and nonglycosylated versions of the energy acceptor, and these are resolved in SDS-PAGE. CHO, carbohydrate; CO-IP, co-immunoprecipitation; IB, immunoblot.