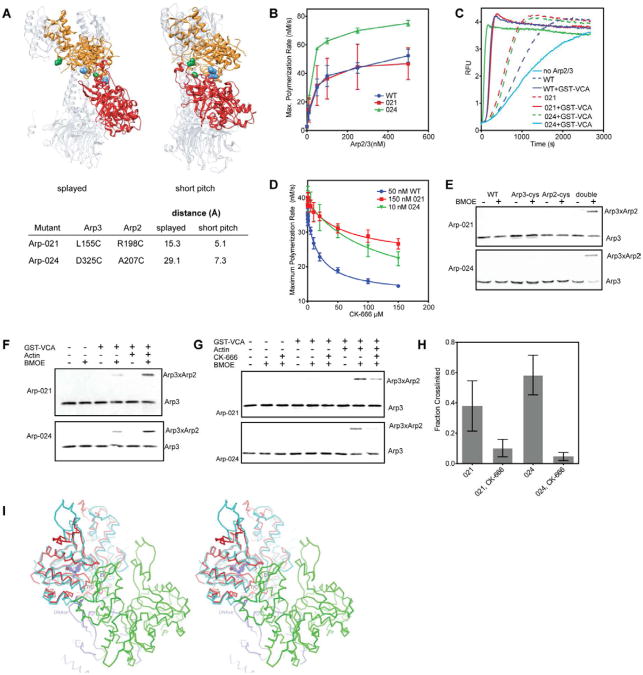
Fig. 5.
CK-666 blocks formation of the short pitch Arp2-Arp3 dimer. (A) Structures of inactive (“splayed”, 2P9K) and active (“short-pitch”)(Rouiller et al., 2008) Arp2/3 complex showing relative orientation of Arp3 (orange) and Arp2 (red). Distance between engineered cysteine residues in mutants Arp-021 (green residues) and Arp-024 (blue atoms) in each conformation is indicated in table. (B) Time courses of actin polymerization containing 3 μM 15% pyrene actin and 150 nM GST-VCA with a range of concentrations of wild type or mutant Arp2/3 complex. Error bars are standard deviations from three separate experiments using either two (Arp-024, wt) or three (Arp-021) different preparations of Arp2/3 complex. (C) Time courses of actin polymerization showing activation of mutant and wild type complexes by 150 nM GST-VCA. Concentrations of complex were adjusted to give similar maximum polymerization rates. (wt: 50 nM, Arp-021: 150 nM, Arp-024: 10nM). The “no Arp” reaction contained 150 nM GST-VCA but no Arp2/3 complex. (D) Plot of maximum polymerization rate versus concentration of CK-666. Reactions contained 150 nM GST-VCA plus inhibitor and were otherwise identical to conditions in panel C. IC50 values were as follows: wild type, 20 ± 2 μM; Arp-021, 63 ± 23 μM; Arp-024, 110 ± 35 μM. (E) Both cysteines are required (double) for crosslinked product to form. Anti-Arp3 western blot of crosslinking reactions containing either double cysteine mutant Arp-021 or Arp-024, or the single mutant version of each, plus 10 μM GST-VCA and 10 μM Latrunculin-B actin. (F) Anti-Arp3 western blots of crosslinking reactions containing 1 μM Arp2/3 complex, 100 μM BMOE, 10 μM GST-VCA and 10 μM Latrunculin-B actin, as indicated. (G) Anti-Arp3 western blot of crosslinking reactions containing 10 μM GST-VCA, 10 μM Latrunculin-B actin, and CK-666 as indicated. (H) Quantification of CK-666 inhibition of the short pitch crosslink. The fraction crosslinked was calculated by measuring the ratio of crosslinked to uncrosslinked Arp3. Error bars represent standard error from three replicates. (I) Model showing structural basis for inhibition of Arp2/3 complex by CK-869. Stereo view diagram showing Arp3 (red) from the CK-869-bound Arp2/3 complex structure superposed with an actin subunit (cyan) from a cryo EM actin filament structure (3G37.pdb). CK-869 is shown as ball-and-sticks with purple carbon atoms. “S” marks the sensor loop and “7/C” marks the β7/αC loop in Arp3. “E/F” marks the αE/αF loop in the green actin subunit in the short pitch position relative to Arp3. See also Figure S3.