Abstract
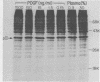
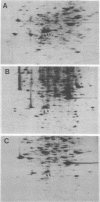
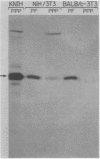
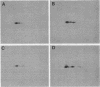
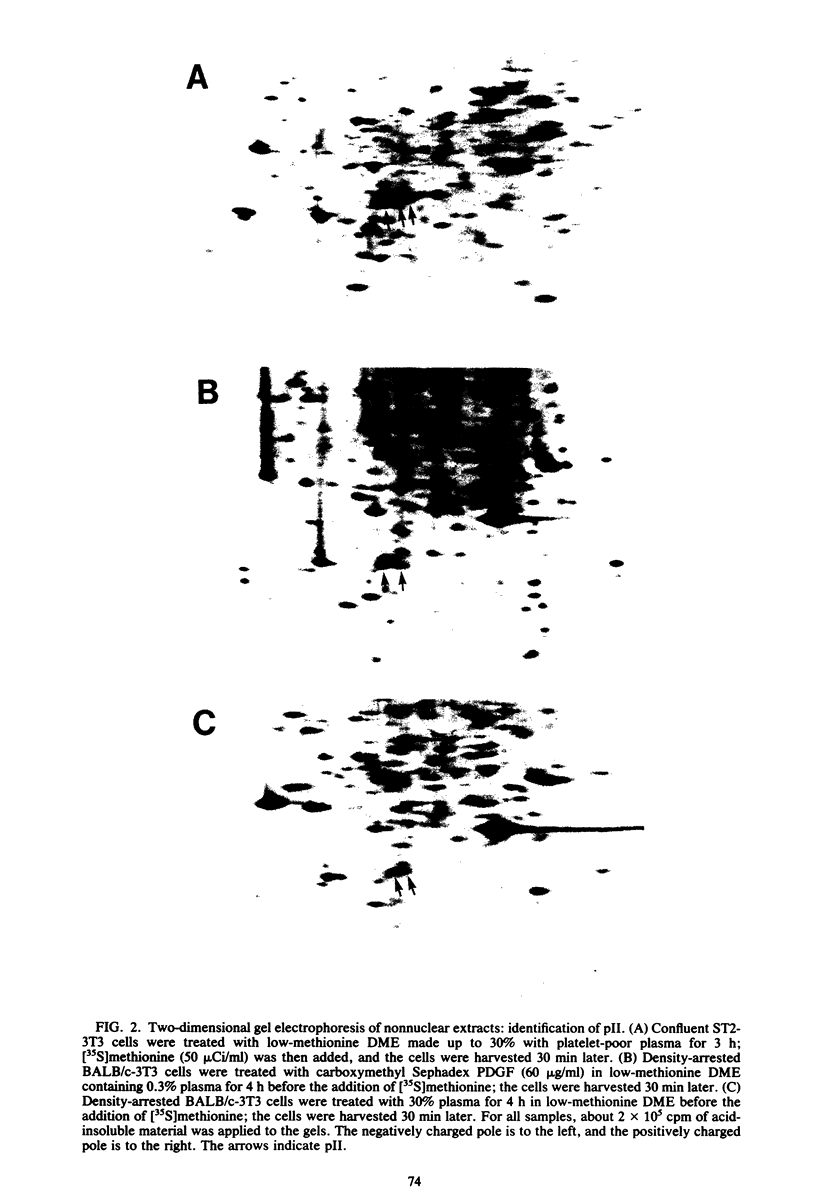
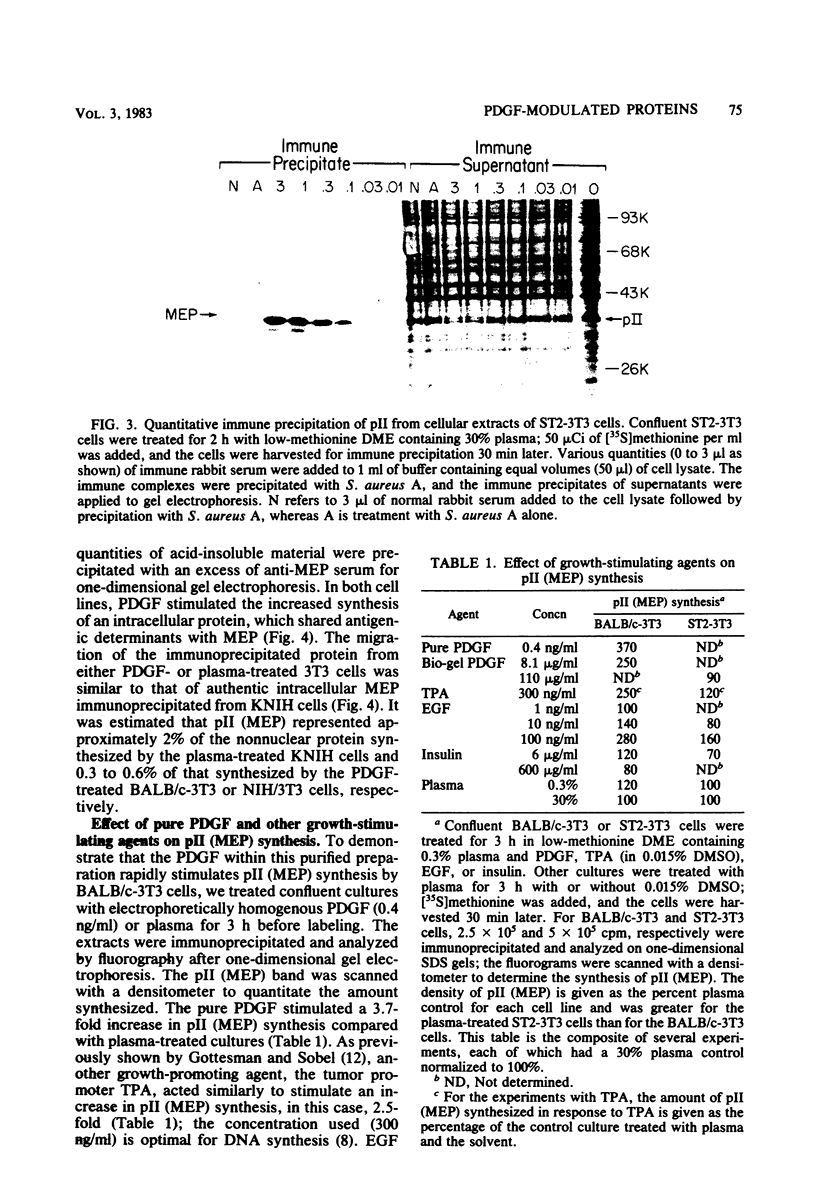
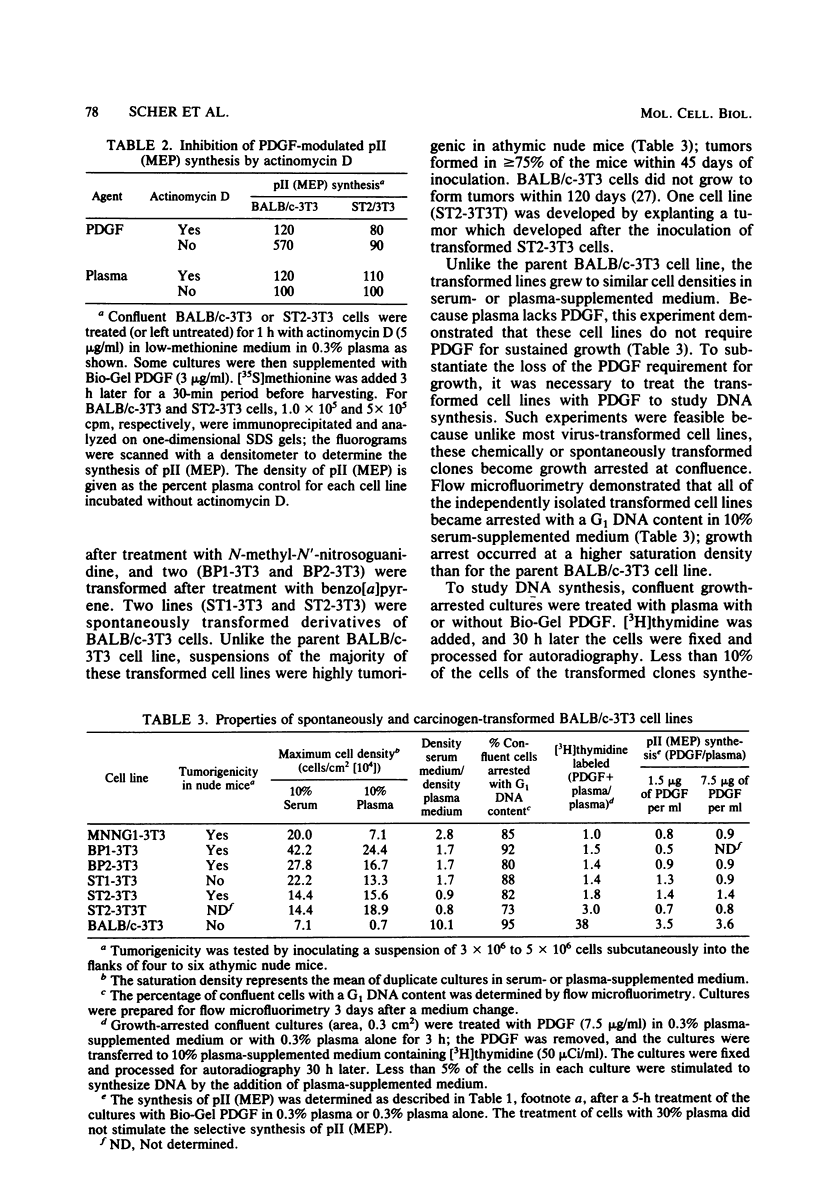
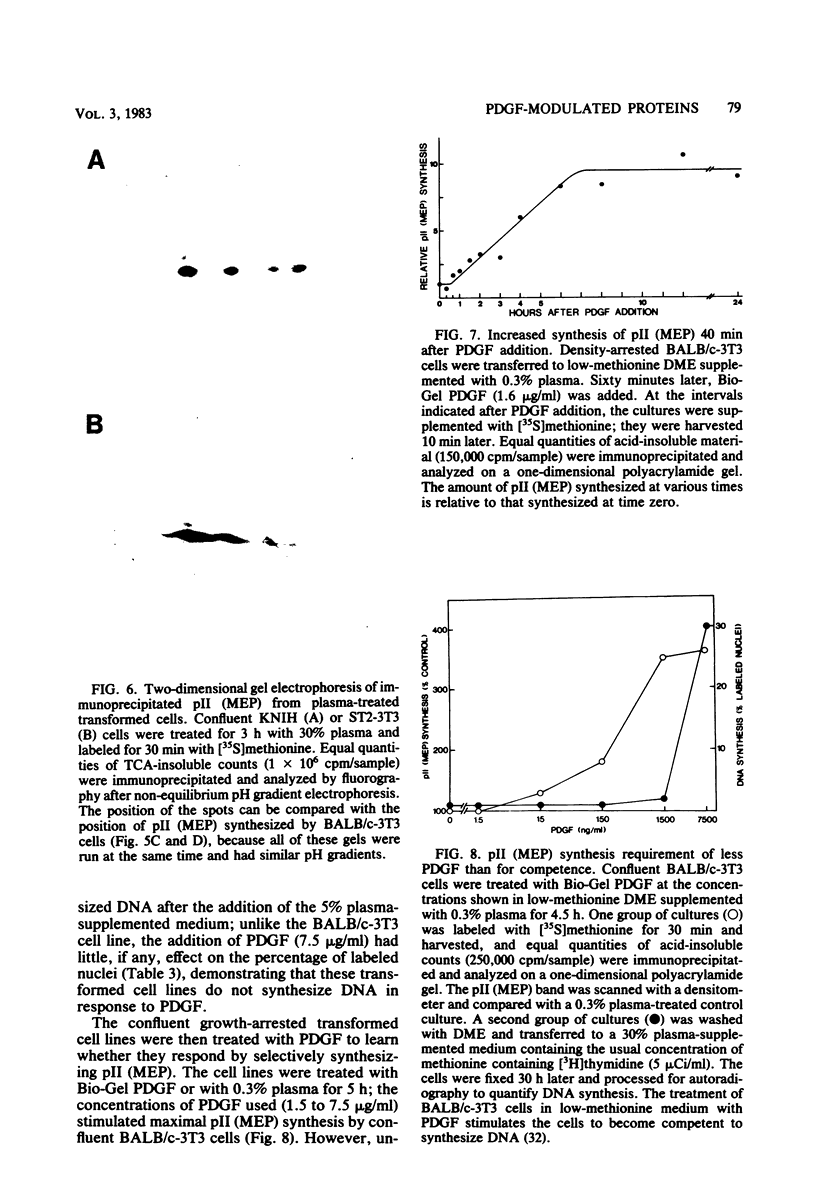
The platelet-derived growth factor (PDGF) stimulates density-arrested BALB/c-3T3 cells to synthesize a protein (pII; Mr, 35,000) that is constitutively synthesized by spontaneously transformed BALB/c-3T3 (ST2-3T3) cells which do not require PDGF for growth. Antisera against a major excreted protein family (MEP) of retrovirus-transformed cells quantitatively precipitated cellular pII. PDGF-stimulated pII has the same molecular weight, a similar charge, and similar antigenic determinants as authentic MEP isolated from ST2-3T3 or retrovirus-transformed cells. MEP represented about 2% of the nonnuclear proteins synthesized by ST2-3T3 cells and 0.3 to 0.6% of the proteins synthesized by PDGF-treated BALB/c-3T3 cells, a three- to sixfold increase over the background. In BALB/c-3T3 cells, less PDGF was required for pII (MEP) synthesis than for DNA synthesis. PDGF induced a selective increase in pII (MEP) within 40 min. Such preferential synthesis was inhibited by brief treatment with actinomycin D, suggesting a requirement for newly formed RNA. The constitutive synthesis of pII (MEP) by ST2-3T3 cells was not inhibited by actinomycin D. Five spontaneously or chemical carcinogen-transformed tumorigenic BALB/c-3T3 cell lines were studied; they neither required PDGF for growth nor responded to it. These cell lines became arrested at confluence with a G1 DNA content. Each of these independently isolated lines synthesized pII (MEP) constitutively. Thus, the synthesis of pII (MEP) may be required, but is not sufficient, for PDGF-modulated DNA synthesis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Scher C. D., Stiles C. D. Purification of human platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1809–1813. doi: 10.1073/pnas.76.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes D., Sato G. Serum-free cell culture: a unifying approach. Cell. 1980 Dec;22(3):649–655. doi: 10.1016/0092-8674(80)90540-1. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cabral F., Gottesman M. M., Yuspa S. H. Induction of specific protein synthesis by phorbol esters in mouse epidermal cell culture. Cancer Res. 1981 Jun;41(6):2025–2031. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Dubrow R., Riddle V. G., Pardee A. B. Different responses to drugs and serum of cells transformed by various means. Cancer Res. 1979 Jul;39(7 Pt 1):2718–2726. [PubMed] [Google Scholar]
- Frantz C. N., Stiles C. D., Scher C. D. The tumor promoter 12-O-tetradecanoyl-phorbol-13-acetate enhances the proliferative response of Balb/c-3T3 cells to hormonal growth factors. J Cell Physiol. 1979 Sep;100(3):413–424. doi: 10.1002/jcp.1041000305. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Bialecki H., Greenburg G. Purification of the fibroblast growth factor activity from bovine brain. J Biol Chem. 1978 May 25;253(10):3736–3743. [PubMed] [Google Scholar]
- Gottesman M. M., Sobel M. E. Tumor promoters and Kirsten sarcoma virus increase synthesis of a secreted glycoprotein by regulating levels of translatable mRNA. Cell. 1980 Feb;19(2):449–455. doi: 10.1016/0092-8674(80)90519-x. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M. Transformation-dependent secretion of a low molecular weight protein by murine fibroblasts. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2767–2771. doi: 10.1073/pnas.75.6.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. M., Yuspa S. H. Tumor promoters induce the synthesis of a secreted glycoprotein in mouse skin and cultured primary mouse epidermal cells. Carcinogenesis. 1981;2(10):971–976. doi: 10.1093/carcin/2.10.971. [DOI] [PubMed] [Google Scholar]
- Holley R. W., Baldwin J. H., Kiernan J. A., Messmer T. O. Control of growth of benzo(a)pyrene-transformed 3T3 cells. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3229–3232. doi: 10.1073/pnas.73.9.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Nilsen-Hamilton M., Hamilton R. T., Allen W. R., Massoglia S. L. Stimulation of the release of two glycoproteins from mouse 3T3 cells by growth factors and by agents that increase intralysosomal pH. Biochem Biophys Res Commun. 1981 Jul 30;101(2):411–417. doi: 10.1016/0006-291x(81)91275-4. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Oshiro Y., DiPaolo J. A. Loss of density-dependent regulation of multiplication of BALB-3T3 cells chemically transformed in vitro. J Cell Physiol. 1973 Feb;81(1):133–138. doi: 10.1002/jcp.1040810116. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Hart C. A., Locatell K. L., Scher C. D. Platelet-derived growth factor-modulated proteins: constitutive synthesis by a transformed cell line. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4358–4362. doi: 10.1073/pnas.78.7.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. An ordered sequence of events is required before BALB/c-3T3 cells become committed to DNA synthesis. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2839–2843. doi: 10.1073/pnas.75.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M., DeStefano M. J. Use of the local anesthetic lidocaine for cell harvesting and subcultivation. In Vitro. 1975 Nov-Dec;11(6):379–381. doi: 10.1007/BF02616374. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Platelet-derived growth factor. I. High yield purification and evidence for multiple forms. J Biol Chem. 1982 May 10;257(9):5154–5160. [PubMed] [Google Scholar]
- Ross R., Vogel A. The platelet-derived growth factor. Cell. 1978 Jun;14(2):203–210. doi: 10.1016/0092-8674(78)90107-1. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Pledger W. J., Martin P., Antoniades H., Stiles C. D. Transforming viruses directly reduce the cellular growth requirement for a platelet derived growth factor. J Cell Physiol. 1978 Dec;97(3 Pt 1):371–380. doi: 10.1002/jcp.1040970312. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Shepard R. C., Antoniades H. N., Stiles C. D. Platelet-derived growth factor and the regulation of the mammalian fibroblast cell cycle. Biochim Biophys Acta. 1979 Aug 10;560(2):217–241. doi: 10.1016/0304-419x(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Singh J. P., Lillquist J. S., Goon D. S., Stiles C. D. Growth factors adherent to cell substrate are mitogenically active in situ. Nature. 1982 Mar 11;296(5853):154–156. doi: 10.1038/296154a0. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Stiles C. D. Cytoplasmic transfer of the mitogenic response to platelet-derived growth factor. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4363–4367. doi: 10.1073/pnas.78.7.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles C. D., Isberg R. R., Pledger W. J., Antoniades H. N., Scher C. D. Control of the Balb/c-3T3 cell cycle by nutrients and serum factors: analysis using platelet-derived growth factor and platelet-poor plasma. J Cell Physiol. 1979 Jun;99(3):395–405. doi: 10.1002/jcp.1040990314. [DOI] [PubMed] [Google Scholar]
- Thimann K. V., Satler S. O. Relation between leaf senescence and stomatal closure: Senescence in light. Proc Natl Acad Sci U S A. 1979 May;76(5):2295–2298. doi: 10.1073/pnas.76.5.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G., Thomas G., Luther H. Transcriptional and translational control of cytoplasmic proteins after serum stimulation of quiescent Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5712–5716. doi: 10.1073/pnas.78.9.5712. [DOI] [PMC free article] [PubMed] [Google Scholar]