Abstract
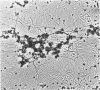
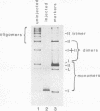
We have noted previously that when circular, but not linear, DNA or chromatin was injected into Xenopus laevis oocytes, much of it went through an intermediate form in which it did not readily enter an agarose gel; after a few hours, it reappeared as monomer DNA that had acquired its full complement of nucleosomes (T. J. Miller and J. E. Mertz, Mol. Cell. Biol. 2:1581-1593, 1982). We determined, using electron microscopy and a variety of biochemical techniques, the structure of this aggregated material. Most of it was oligomeric and multimeric catenanes of the injected sample. In addition, injection of DNA that had been catenated in vitro with DNA gyrase resulted in the conversion of most of it back to monomer circles. These findings demonstrate directly that both catenation and decatenation of DNA occur in vivo under physiological conditions. Whether these reactions play a crucial role in nucleosome formation, as well as in DNA replication and recombination, remains to be determined.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldi M. I., Benedetti P., Mattoccia E., Tocchini-Valentini G. P. In vitro catenation and decatenation of DNA and a novel eucaryotic ATP-dependent topoisomerase. Cell. 1980 Jun;20(2):461–467. doi: 10.1016/0092-8674(80)90632-7. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R. DNA gyrase and the supercoiling of DNA. Science. 1980 Feb 29;207(4434):953–960. doi: 10.1126/science.6243420. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R. DNA topoisomerases. Cell. 1980 Nov;22(2 Pt 2):327–328. doi: 10.1016/0092-8674(80)90341-4. [DOI] [PubMed] [Google Scholar]
- Gandini Attardi D., Martini G., Mattoccia E., Tocchini-Valentini G. P. Effect of Xenopus laevis oocyte extract on supercoiled simian virus 40 DNA: formation of complex DNA. Proc Natl Acad Sci U S A. 1976 Feb;73(2):554–558. doi: 10.1073/pnas.73.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Germond J. E., Hirt B., Oudet P., Gross-Bellark M., Chambon P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci U S A. 1975 May;72(5):1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T., Brutlag D. ATP-dependent DNA topoisonmerase from D. melanogaster reversibly catenates duplex DNA rings. Cell. 1980 Aug;21(1):115–125. doi: 10.1016/0092-8674(80)90119-1. [DOI] [PubMed] [Google Scholar]
- Krasnow M. A., Cozzarelli N. R. Catenation of DNA rings by topoisomerases. Mechanism of control by spermidine. J Biol Chem. 1982 Mar 10;257(5):2687–2693. [PubMed] [Google Scholar]
- Kreuzer K. N., Cozzarelli N. R. Formation and resolution of DNA catenanes by DNA gyrase. Cell. 1980 May;20(1):245–254. doi: 10.1016/0092-8674(80)90252-4. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Liu C. C., Alberts B. M. Type II DNA topoisomerases: enzymes that can unknot a topologically knotted DNA molecule via a reversible double-strand break. Cell. 1980 Mar;19(3):697–707. doi: 10.1016/s0092-8674(80)80046-8. [DOI] [PubMed] [Google Scholar]
- Mattoccia E., Attardi D. G., Tocchini-Valentini G. P. DNA-relaxing activity and endonuclease activity in Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4551–4554. doi: 10.1073/pnas.73.12.4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz J. E. Linear DNA does not form chromatin containing regularly spaced nucleosomes. Mol Cell Biol. 1982 Dec;2(12):1608–1618. doi: 10.1128/mcb.2.12.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. J., Mertz J. E. Template structural requirements for transcription in vivo by RNA polymerase II. Mol Cell Biol. 1982 Dec;2(12):1595–1607. doi: 10.1128/mcb.2.12.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. J., Stephens D. L., Mertz J. E. Kinetics of accumulation and processing of simian virus 40 RNA in Xenopus laevis oocytes injected with simian virus 40 DNA. Mol Cell Biol. 1982 Dec;2(12):1581–1594. doi: 10.1128/mcb.2.12.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. L., Miller T. J., Silver L., Zipser D., Mertz J. E. Easy-to-use equipment for the accurate microinjection of nanoliter volumes into the nuclei of amphibian oocytes. Anal Biochem. 1981 Jul 1;114(2):299–309. doi: 10.1016/0003-2697(81)90485-1. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Laskey R. A., Finch J., Gurdon J. B. Selective DNA conservation and chromatin assembly after injection of SV40 DNA into Xenopus oocytes. Dev Biol. 1978 May;64(1):178–188. doi: 10.1016/0012-1606(78)90069-6. [DOI] [PubMed] [Google Scholar]