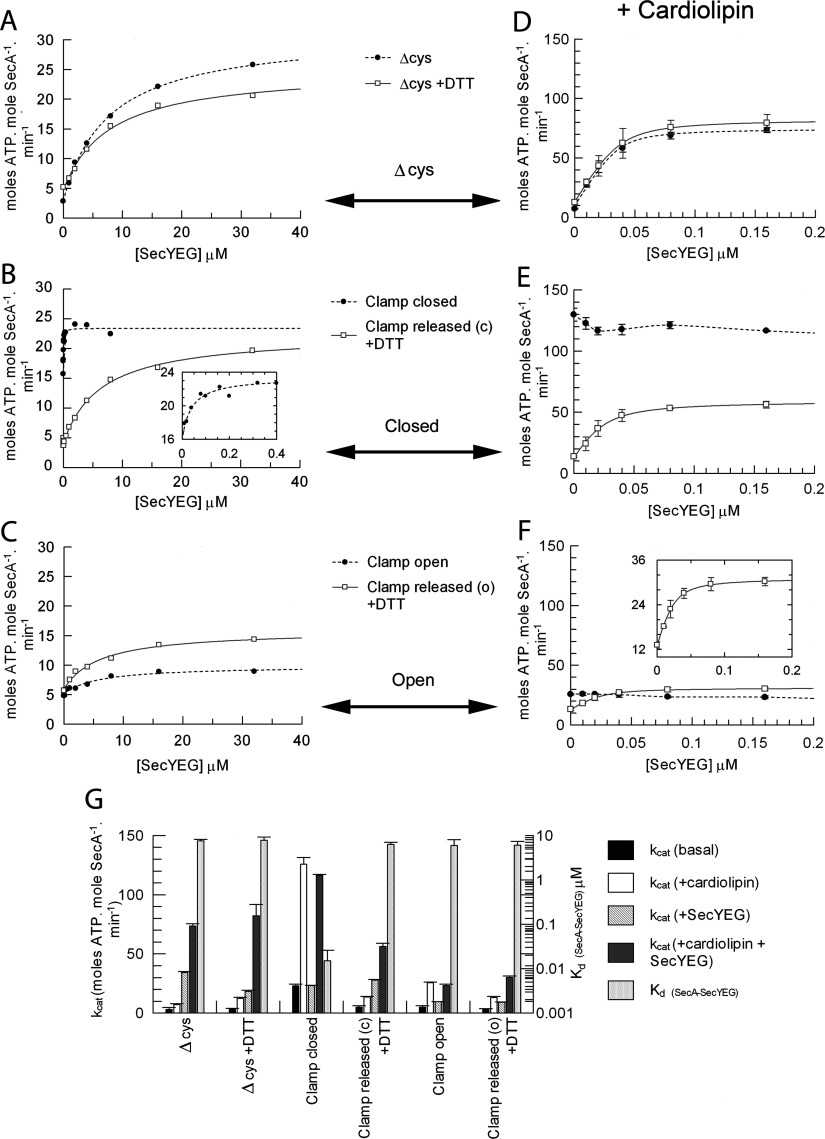
Figure 2. Analysis of SecA ATPase activity and binding affinity for SecYEG.
Steady-state ATPase activity of 0.15 μM SecA in TKM buffer in the presence of 1 mM ATP and increasing concentrations of purified detergent-solubilized SecYEG. Data were fitted to a ligand-binding equation and the parameters are shown in Supplementary Table S1 (at http://www.BiochemJ.org/bj/449/bj4490695add.htm), columns 1 and 2. (A) SecAΔcys in the absence (broken trace) and presence (continuous trace) of 10 mM DTT. (B) SecAD337C/K482C cross-linked with the clamp closed (broken trace; no DTT) and with the clamp released from the closed (c) state (continuous trace; 10 mM DTT). (C) SecAD337C/E806C cross-linked with the clamp open (broken trace; no DTT) and with the clamp released from the open (o) state (continuous trace; 10 mM DTT). (D–F) Experiments shown in (A–C) were repeated in the presence of 40 μM CL. (G) The end point kcat values under the conditions described, along with the Kd of SecA binding SecYEG, are shown in the histogram (also in Supplementary Table S1, columns 3 and 4).