Abstract
While many prognostic markers in B-cell chronic lymphocytic leukemia provide insight into the biology of the disease, few have been demonstrated to be useful in the daily management of patients. B-cell receptor signaling is a driving event in the progression of B-cell chronic lymphocytic leukemia and markers of B-cell receptor responsiveness have been shown to be of prognostic value. Single cell network profiling, a multiparametric flow cytometry-based assay, allows functional signaling analysis at the level of the single cell. B-cell receptor signaling proteins (i.e. p-SYK, p-NF-κB p65, p-ERK, p-p38, p-JNK) were functionally characterized by single cell network profiling in samples from patients with B-cell chronic lymphocytic leukemia in an exploratory study (n=27) after stimulation with anti-IgM. Significant associations of single cell network profiling data with clinical outcome (i.e. time to first treatment), as assessed by Cox regression models, were then confirmed in patients' samples in two other sequential independent studies, i.e. test study 1 (n=30), and test study 2 (n=37). In the exploratory study, higher responsiveness of the B-cell receptor signaling proteins to anti-IgM was associated with poor clinical outcomes. Patients' clustering based on signaling response was at least as powerful in discriminating different disease courses as traditional prognostic markers. In an unselected subgroup of patients with Binet stage A disease (n=21), increased anti-IgM-modulated p-ERK signaling was shown to be a significant, independent predictor of shorter time to first treatment. This result was independently confirmed in two test cohorts from distinct populations of patients. In conclusion, these findings support the utility of the single cell network profiling assay in elucidating signaling perturbations with the potential for the development of a clinically useful prognostic test in patients with early stage B-cell chronic lymphocytic leukemia. These data support the clinical relevance of B-cell receptor signaling in B-cell chronic lymphocytic leukemia, and suggest a key role of ERK activation in the physiopathology of this leukemia.
Introduction
Several biological parameters have been shown to be associated with clinical outcomes in patients with B-cell chronic lymphocytic leukemia (B-CLL) and are used individually, in combination, or as part of prognostic nomograms to stratify patients into those with a more indolent course not requiring therapy for a prolonged period of time versus those with a more aggressive form of the disease and a reduced time to first treatment (TTFT).1-3 These parameters include the presence or absence of: (i) somatic mutations within the immunoglobulin variable heavy chain genes (IGHV); (ii) specific chromosomal abnormalities; (iii) the expression of the ZAP-70 tyrosine kinase in the cytosol and (iv) the expression of CD38 surface antigen.4 However, there remains substantial intragroup clinical heterogeneity in otherwise molecularly homogeneous B-CLL subgroups, particularly among patients with early stage disease.5 Although the pathogenesis of B-CLL and the reason for this outcome disparity are still not fully understood, there is strong evidence that B-cell receptor (BCR) activation and signaling is a driving event in the onset and progression of B-CLL.6 BCR stimulation induces an increase of intracellular calcium, global protein tyrosine phosphorylation and activation of proteins downstream of the BCR signaling pathways, i.e. spleen tyrosine kinase (SYK), extracellular signal-regulated kinase (ERK), and serine/threonine-protein kinase AKT.7 Signaling events downstream of the BCR are heterogeneous among B-CLL patients and it has been hypothesized that differences in antigen-induced BCR activity may determine the variable clinical behavior of B-CLL. Indeed, B-CLL cells with unmutated IGHV genes display a high responsiveness to BCR stimulation, which leads to increased proliferation and survival, and is associated with aggressive disease. By contrast, B-CLL cells with mutated IGHV genes and indolent disease show a weaker and less frequent BCR responsiveness.7
Several lines of evidence support the importance of BCR signaling to clinical outcome.8 A recent report demonstrated decreased progression-free survival and overall survival in patients with in-vitro findings of BCR-induced B-CLL survival via NFAT2.9 In addition, a strong association was found between BCR-induced phosphorylation of the proapoptotic protein BIM and progression of B-CLL.10 Furthermore, in IGHV unmutated B-CLL signaling through the BCR induced telomerase activity and promoted cell survival.11 Finally, the importance of BCR signaling in B-CLL has been recently further highlighted by promising clinical results obtained using therapeutic agents that directly target elements of BCR signaling.12,13
Despite the clear importance of BCR signaling in the outcomes of patients with B-CLL, there is no easily available practical test that translates the above findings from bench to bedside use in the management of patients with B-CLL.
Single cell network profiling (SCNP) is a multi-parametric flow cytometry-based assay that measures, simultaneously and quantitatively, at the single cell level, both extracellular surface marker levels and changes in intracellular signaling proteins in response to extracellular modulators.14-16 The association of SCNP data with in vivo acute myeloid leukemia (AML) chemosensitivity/chemoresistance17 as well as FLT3R signaling deregulation18 and applications in immunology19,20 have been previously reported.
Here the SCNP assay was first applied in an exploratory study to functionally characterize elements of the BCR signaling network and to assess their association with clinical and prognostic parameters with the goal of ultimately developing a clinically useful tool for the prediction of B-CLL progression and early (<3 years) TTFT in patients with early stage B-CLL. The prognostic impact of p-ERK response to anti-IgM was independently confirmed in two test cohorts.
Design and Methods
Donors' samples
For the exploratory and first test cohorts peripheral blood mononuclear cell samples were collected and cryopreserved at the Hematology Unit, Azienda Ospedaliera Universitaria Integrata (AOUI) in Verona (Italy). In the first study (exploratory), peripheral blood mononuclear cell samples from B-CLL patients (n=27) were analyzed at the laboratory of Verona University (Verona, Italy). (See Online Supplementary Figure S1 for a schema of the studies). In the second study (test 1), peripheral blood mononuclear cells from previously-untreated B-CLL patients (n=30) were analyzed at Nodality's laboratory in South San Francisco (CA, USA). To confirm assay reproducibility between laboratories, a “bridging” study was performed prior to the test studies, using ten duplicate B-CLL patients' samples already tested in the exploratory study for which additional aliquots were available. For verification using a population of patients distinct from the other cohorts (test 2), peripheral blood mononuclear cells were collected and cryopreserved at the Feinstein Institute for Medical Research, North Shore Long Island Jewish Health System, New York (USA) from a cohort of 37 previously untreated B-CLL patients at different times from diagnosis. SCNP assays were performed blinded to clinical outcomes.
B-CLL patients' samples were collected under a protocol approved by the local Ethics Committee or Institutional Review Board. In accordance with the Declaration of Helsinki, all patients provided written informed consent for the collection and use of their blood samples for research purposes. Diagnosis and initiation of treatment for B-CLL were based on 1996 National Cancer Institute-Working Group (NCI-WG)/IWCLL and 2008 Guidelines for Diagnosis and Treatment of CLL.21,22 B-CLL patients and sample eligibility criteria are described in Online Supplementary Table S1. In the exploratory study, samples from patients with all stages of B-CLL were analyzed, while in the two test studies, the analysis was limited to either Binet Stage A or Rai Stage 0 and I B-CLL patients' samples, since these subgroups represent the patients who will benefit most from an improved prognostic marker. Clinical and biological disease characteristics at diagnosis as well as clinical outcomes of the B-CLL patients in the exploratory and test sets are summarized in Online Supplementary Table S2.
In all studies, ZAP-70 and CD38 were determined using cut-offs of 20% and 30%, respectively23 and IGHV sequencing utilized a 2% cut-off to descriminate unmutated (UM) from mutated (M) IGHV.
Experimental methodology
The exploratory study samples were processed for the SCNP assay in Verona University's laboratory, while the bridging and test studies and all data analyses were performed at Nodality. All studies followed the general experimental methods described previously.16-17,24 Key differences between the methodologies at Verona University and Nodality are summarized in Online Supplementary Table S3 and detailed in the Online Supplementary Design and Methods.
Details on the exploratory and gating analysis in the exploratory and test studies are also provided in the Online Supplementary Design and Methods.
Single cell network profiling assay terminology and metrics
The term “signaling node” or simply “node” is used to refer to a proteomic readout in the presence or absence of a specific modulator. For example, the response to anti-IgM modulation can be measured using p-ERK as a readout. That signaling node is designated “anti-IgM→p-ERK”. The term “metric” is used to refer to the quantification method used to evaluate the functional response of signaling proteins. The log2Fold metric measures the magnitude of the responsiveness of a cell population to modulation relative to the same cell population in the reference well (e.g., isotype or unmodulated) by comparing the median fluorescence values of the responsive cell population to that of the reference population on a log2 scale. A value of zero would indicate overlapping populations and a value different from zero indicates the responsive population has shifted to higher fluorescence (positive values) or to lower fluorescence (negative values). The log2Fold metric is calculated as log2(RMFI modulated/RMFI unmmodulated) in the exploratory study, and log2(ERF modulated/ERF unmodulated) in the test studies. The Uu metric is the Mann-Whitney U statistic that compares the ERF values of the modulated and unmodulated wells that have been scaled to the unit interval (0,1) for a given donor and quantifies the fraction of cells responding to a specific modulation.24
When combined, a “node-metric” is a quantified change in signal and is used to interpret the functionality and biology of each signaling node. It is annotated as “node | metric”, e.g. “anti-IgM→p-ERK | log2Fold”.
Data analysis in the exploratory study
The two-sample Wilcoxon's rank sum test was used to compare phosphorylation of BCR signaling proteins in groups of patients. Differences between the data were considered statistically significant for P values <0.05. K-medoids clustering according to the Partitioning Around Medoids algorithm implemented in the pam function of the R cluster package version 1.13.1 was used for grouping patients' samples on the basis of anti-IgM antibody modulated BCR network signaling response. Fisher's exact test was used to assess the concordance between signaling-defined groups of patients and strata based on currently used prognostic factors (such as IGHV mutational status and CD38 and ZAP-70 expression status).
To determine BCR signaling element connectivity the relationships between responses of signaling proteins were examined by calculating correlation coefficients for the log2Fold change SCNP data pairwise combinations.
To evaluate the prognostic significance of the SCNP-defined grouping of patients, TTFT curves estimated using the Kaplan-Meier method for the respective groups of patients were compared using the log-rank test. Furthermore, the SCNP-based prognostic groups were compared (using the log-rank test) to their respective prognostic groups defined by IGHV, ZAP-70, or CD38 status. For these comparisons as well as the modeling described in the following sections, TTFT was calculated from the date of diagnosis to the date of initial therapy21,22 in all studies. Cases were censored when treatment was not initiated prior to the last follow-up, in which case the censoring date was the date of last follow-up. The median TTFT and follow-up times were estimated using the Kaplan-Meier method.
Univariate and bivariate models for TTFT were generated using Cox proportional hazards regression implemented in the rms package version 3.1-0 of the R software. Inputs to the models were the change in phosphorylation for each BCR network protein in response to anti-IgM (expressed as log2Fold change), standard of practice prognostic markers, and clinical covariates. Categorical covariates were coded as 0 or 1 as follows: IGHV mutated = 0, IGHV unmutated = 1; ZAP-70 negative = 0, ZAP-70 positive = 1; CD38 negative = 0, CD38 positive = 1. Bivariate analysis included all possible pairs of inputs to the univariate models. Operating characteristics of the time to event models were summarized using both the likelihood ratio χ2 (LR) and Harrell's concordance index (C), which assesses how well a model orders patients in terms of TTFT25 In the exploratory study, models were selected only if the P-values for each coefficient and the model LR χ2 were <0.10 (this cut-off was chosen on the basis of the small sample size and on the “hypothesis generating” nature of the study). All statistical analyses were performed using the R statistical programming package.26
Model verification analysis in the test studies
The association between increased anti-IgM→p-ERK signaling and shorter TTFT was pre-specified and tested in the test studies by constructing a Cox proportional hazards model for TTFT using the anti-IgM→p-ERK | log2Fold or anti-IgM→p-ERK | Uu change as a predictor of TTFT. The association was considered statistically significant if the P-value for the LR χ2 for the model was <0.05.
Results
Exploratory study
Characteristics of the patients and samples
Twenty-seven samples from patients with B-CLL were evaluated in the exploratory study (Online Supplementary Figure S1). The characteristics of the B-CLL patients and samples are summarized in Online Supplementary Table S2. Twenty-one samples were from patients with Binet stage A disease, and these were used to model TTFT. Of note, although classified as Binet stage A, six of the 21 samples (29%) were collected from Binet stage A patients who had previously received treatment (at least 6 months prior to sample collection). During the course of follow-up (median 164 months), 14 Binet stage A patients became symptomatic, necessitating treatment, for an event rate of 67% and a median TTFT of 50 months.
Differential B-cell receptor signaling responses and network connectivity in subsets of B-cell chronic lymphocytic leukemia
In the exploratory study, the phosphorylation levels of five phosphoproteins downstream of the BCR signaling, namely p-SYK, p-NF-κB p65, p-ERK, p-p38 and p-JNK, were analyzed at the single-cell level by SCNP in the 27 B-CLL cell samples. The phosphorylation of the BCR signaling proteins was measured in the basal (i.e. unmodulated) condition and following anti-IgM stimulation (i.e. anti-IgM-modulated condition).
In the basal condition, BCR signaling varied among samples, ranging from a clear positive fluorescence in at least one of the nodes measured to no change with respect to the isotype (Figure 1A,B). However, no significant differences were detected between basal BCR protein phosphorylation in subgroups of patients defined by IGHV mutational status (data not shown).
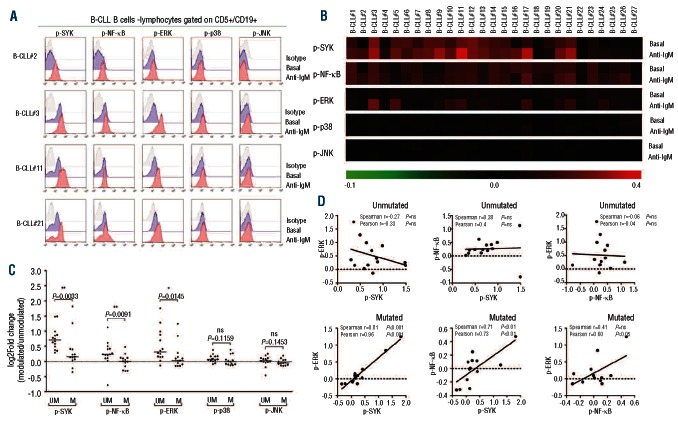
Figure 1.
BCR signaling profiles in B cells from B-CLL patients – exploratory study. (A) Representative flow cytometry histograms of BCR signaling phosphoproteins in basal conditions or following stimulation with anti-IgM in CD5+/CD19+ cells from B-CLL patients. (B) BCR signaling profiles in basal condition or following stimulation with anti-IgM across B-CLL samples. Data are expressed as log2Fold difference in phosphoprotein median fluorescence intensity (MFI) divided by isotype-matched control (relative median fluorescence intensity = RMFI) and represented as a pseudo-color map. (C) Response to BCR modulation in unmutated (UM) and mutated (M) IGHV samples. Data are expressed as log2Fold difference in RMFI of anti-IgM-modulated divided by unmodulated cells (log2Fold change). The line in the middle represents the median value. The B-CLL subsets were confirmed by the two Wilcoxon's rank sum test. *P<0.05; **P<0.01; ns = not significant. (D) Correlation between pairwise combinations of phosphoprotein response to anti-IgM, measured as log2Fold change, in unmutated and mutated B-CLL subsets.
The BCR signaling in response to anti-IgM, as measured by SCNP as the log2Fold change in phosphorylation between anti-IgM-modulated and unmodulated conditions, varied among B-CLL patients (Figure 1B,C), showing significantly higher responses for p-SYK, p-NF-κB and p-ERK in the UM versus the M subset of B-CLL (Figure 1C). In addition, the proportion of samples showing a positive response to anti-IgM stimulation (fold change ≥ 1.2) was higher in the UM than in the M B-CLL group (Online Supplementary Table S5), thus confirming and extending previous findings.8 In contrast, no statistically significant differences were observed between the UM and M subsets for p-p38 and p-JNK responses to anti-IgM (Figure 1C).
The relationships between responses of signaling proteins that had showed quantitative differences between the two B-CLL prognostic subsets (i.e. SYK, NF-κB and ERK) were then examined by calculating correlation coefficients for the log2Fold change SCNP data pairwise combinations. As shown in Figure 1D, in the UM B-CLL subset, responses of signaling proteins appeared unrelated to each other, with the exception of SYK and ERK responses, which were inversely correlated. In contrast, in the M B-CLL subset signaling protein responses were positively correlated with each other (Figure 1D).
The differential BCR signaling responses and connectivity of the signaling network proteins between the major B-CLL prognostic subsets suggest that BCR signaling, as measured by the SCNP assay, could differentiate prognostic classes of B-CLL patients.
Association of B-cell chronic lymphocytic leukemia B-cell receptor signaling profiles with biological parameters and clinical progression
BCR responses, measured by the log2Fold change in phosphorylation between anti-IgM-modulated and unmodulated conditions, were subjected to unsupervised clustering analysis within an unselected group of Binet stage A B-CLL patients. SCNP data identified two main clusters of B-CLL patients: cluster 1 included samples that showed a greater signaling response to BCR engagement, whereas cluster 2 comprised samples with a lower signaling response (Figure 2A).
Figure 2.
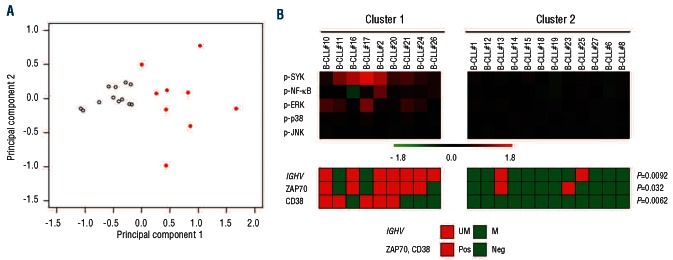
Binet stage A B-CLL patients grouped on the basis of responsiveness to BCR modulation were associated with biological prognostic parameters – exploratory study. Response to BCR engagement was expressed as log2Fold difference in relative median fluorescence intensity (RMFI) of anti-IgM-modulated cells divided by unmodulated cells (log2Fold change). Patients were then grouped based on BCR log2Fold changes using the k-medoids clustering algorithm. (A) Scatter plot in the first two principal components of BCR response to anti-IgM. Each point represents a patient, the pattern or filled quadrants denotes cluster membership: empty indicates cluster 2, filled and red indicates cluster 1. (B) B-CLL patient clusters are represented as a pseudo-color map. The standard biological parameters of prognosis are aligned below the heat map.
The two B-CLL clusters defined by the BCR signaling response were then considered in relation to IGHV, ZAP-70, and CD38 statuses. Fisher's test showed significant concordance between the signaling-based classification and IGHV, ZAP-70, and CD38 statuses (P=0.0092, P=0.032, P=0.0062, respectively) (Figure 2B).
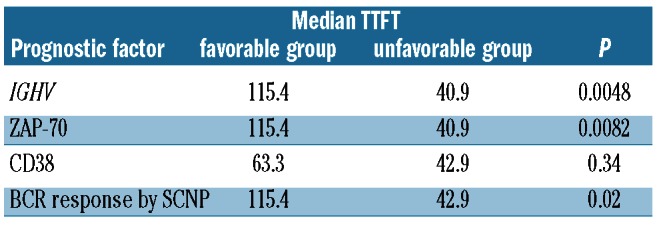
The relationships between the BCR responsiveness profiles and TTFT were then examined. Table 1 shows the median TTFT was 43 months for patients in cluster 1 (unfavorable prognostic group) and 115 months for patients in cluster 2 (favorable prognostic group; log-rank χ2 test P=0.02). The median TTFT for the respective prognostic clusters defined by BCR response signaling were very similar to those of the groups defined by the prognostic molecular markers IGHV and ZAP-70; in contrast, CD38 showed no significant differences in TTFT (Table 1). The ability of BCR responsiveness signaling to define prognostic groups is comparable to that of IGHV mutational status, ZAP-70 or CD38, since the Kaplan-Meier curves obtained using the BCR responsiveness signaling showed no significant differences compared with those obtained using IGHV mutational status (log-rank test P=0.64 and P=0.80 for the unfavorable and favorable prognostic groups, respectively) (Online Supplementary Figure S4), ZAP-70 (P=0.95 and P=0.81 for the unfavorable and favorable prognostic groups, respectively) (data not shown) or CD38 (P=0.58 and P=0.67 for the unfavorable and favorable prognostic groups, respectively) (data not shown).
Table 1.
Median TTFT in prognostic groups defined by classical prognostic factors and SCNP.
Univariate and multivariate analysis of association of B-cell receptor-modulated signaling nodes with clinical outcomes (time to first treatment)
The clustering results suggested that SCNP data contained relevant prognostic information; however, clustering methods are not suitable for prospective classifier development because adding new samples to the data set may change the cluster definitions leading to potentially inconsistent classification of the same patient over time. Therefore, time-to-event modeling utilizing both SCNP data and currently used prognostic factors (i.e., IGHV mutational status, CD38 and ZAP-70 expression) alone and in combination was investigated. Six Cox regression models met the significance criteria described in the methods for the exploratory study. These models and their performance characteristics are presented in Table 2A.
Table 2A.
Summary of univariate and multivariate models for TTFT generated in the exploratory study using all Binet stage A patients (n=21; log2Fold metric).
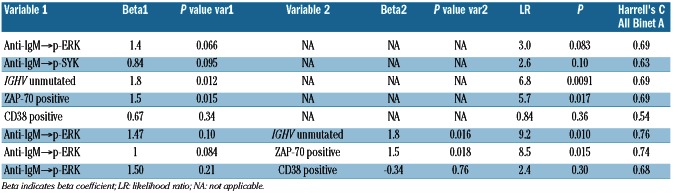
In this set of patients, univariate time-to-event analysis identified increased anti-IgM→p-ERK | log2Fold (LR χ2 test P=0.083; Table 2A; Figure 3A), increased anti-IgM→p-SYK (LR χ2 test P=0.10), IGHV unmutated status (LR χ2 test P=0.009), and ZAP-70 positivity (LR χ2 test P=0.017) as significant, independent predictors of shorter TTFT (Table 2A). Of note, CD38 cell surface expression levels were not a significant predictor in this sample set. In a multivariate time-to-event analysis, two models combining anti-IgM→p-ERK | log2Fold with IGHV unmutated status or with ZAP-70 positivity showed significance (Table 2A).
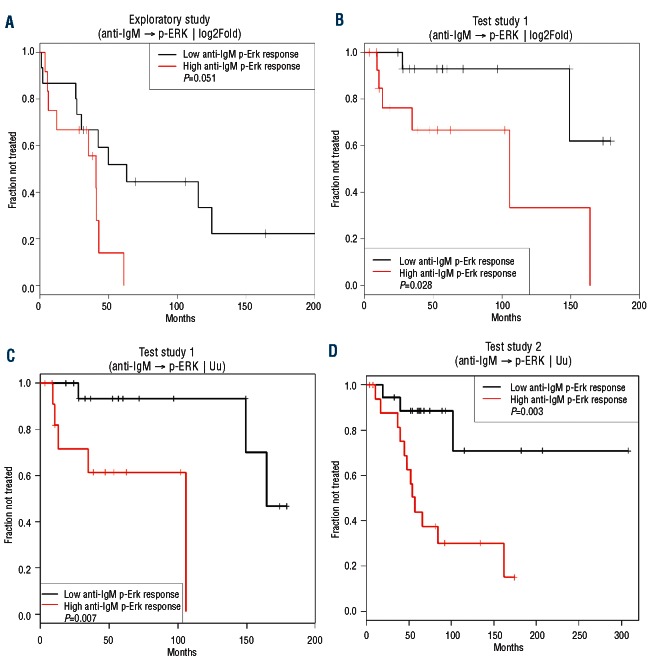
Figure 3.
Kaplan-Meier curves of TTFT for subgroups of Binet stage A or Rai I/0 patients defined by p-ERK response to anti-IgM in the exploratory study (A); test study 1 using the anti-IgM→p-ERK | log2Fold (B) or the anti-IgM→p-ERK | Uu metric (C); test study 2 (D). High and low p-ERK values were referred to the median signal values in (A) and (B) and to the 0.66 cut-point for the Uu metric (see the Online Supplementary Design and Methods section). P values are from the log-rank test.
These results suggest that SCNP might provide complementary information to the currently available B-CLL prognostic tests, a hypothesis that requires testing in an independent set of patient samples.
Test study 1
Characteristics of the patients and samples
After a bridging study between the University of Verona and Nodality laboratories confirmed concordant results between the two laboratories (see the Online Supplementary Design and Methods and Online Supplementary Figure S5), the external validity of the association between anti-IgM→p-ERK and TTFT was tested in an independent set of samples from patients with Binet stage A B-CLL (n=30) at the Nodality laboratory. Although the test and exploratory study populations had similar molecular prognostic markers, the test study population was characterized by statistically significantly shorter follow-up (median 57 months in the test set versus 164 months for the exploratory set; P=0.049), with only 27% of test study patients having developed active disease requiring treatment during the observation period (versus 67% in the exploratory set; P=0.011) (Online Supplementary Table S2). Moreover, the median TTFT was estimated to be 149 months in the test study as compared to 50 months in the exploratory study (median TTFT estimated using the Kaplan-Meier method; P=0.059) (Online Supplementary Table S2).
Association of anti-IgM→p-ERK with clinical progression in the test study 1
Consistent with the results of the exploratory study, a statistically significant association between increased anti-IgM→p-ERK and shorter TTFT was observed in the test study 1 using either the log2Fold (LR χ2 test P=0.026; Table 2B) or Uu metric (LR χ2 test P=0.032; Table 2C). By contrast, an association between anti-IgM→p-SYK and shorter TTFT did not meet the significance criteria specified for the test studies (Table 2B-C). Of the current clinical molecular prognostic markers of TTFT, in this test set IGHV mutational status (LR χ2 test P=0.0005) and CD38 (LR χ2 test P=0.03), but not ZAP70, showed a significant association with TTFT. In a multivariate time-to-event analysis, two models combining anti-IgM→p-ERK | log2Fold or | Uu with IGHV unmutated status or with CD38 positivity showed significance (Table 2B-C).
Table 2B.
Selected Cox proportional hazards models for TTFT in the test study 1 (n=30; log2Fold metric).
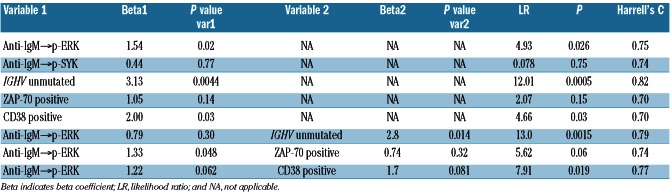
Table 2C.
Selected Cox proportional hazards models for TTFT in the test study 1 (n=30; Uu metric).
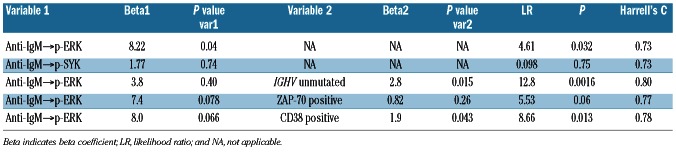
In this set of patients, a significant difference in TTFT was observed in Kaplan-Meier curves between samples from higher and lower anti-IgM-pERK responders using either the log2Fold (log-rank χ2 test P=0.028; Figure 3B) or the Uu metric (log-rank χ2 test P=0.007; Figure 3C) applying the 0.66 cutpoint (see the Online Supplementary Design and Methods section). The Uu metric, which gave the lowest log-rank χ2 test P-value, was used to test the association between anti-IgM→p-ERK response and TTFT in a second independent set of patients' samples.
Test study 2
Characteristics of the patients and samples
Test study 2 consisted of samples from patients with Rai stage 0 or I B-CLL (n=37). These patients were more similar in both age at diagnosis (median 56 years) and at time of sample collection (median 58 years) to those in the exploratory study than to those in test study 1. Similar to test study 1 there was a male preponderance (76%). There were no statistically significant differences in molecular prognostic markers between the three studies. At the time of SCNP analysis, 15 patients (41%) had progressed, requiring treatment. The median follow-up was 102 months (range, 11-162 months) (Online Supplementary Table S2).
Association of anti-IgM→p-ERK | Uu with clinical progression in test study 2
The significant association between increased anti-IgM→p-ERK signaling and shorter TTFT observed in the exploratory study and test study 1 was further confirmed in test study 2 (LR χ2 test P=0.027; Table 2D; Figure 3D). BCR-modulated p-SYK also showed an association with TTFT (LR χ2 test P=0.014). Of the current clinical molecular prognostic markers of TTFT, in this test set IGHV mutational status (LR χ2 test P=0.013) and CD38 (LR χ2 test P=0.037) but not ZAP70 showed a significant association with TTFT. In a multivariate time-to-event analysis, the models combining anti-IgM→p-ERK | Uu with IGHV unmutated status, ZAP-70 or CD38 positivity showed significance (Table 2D). Remarkably, combining anti-IgM→p-ERK | Uu with ZAP-70 positivity improved the significance of the ZAP-70 association with TTFT (P=0.33 to P=0.037) (Table 2D).
Table 2D.
Selected Cox proportional hazards models for TTFT in test study 2 (n=37; Uu metric).
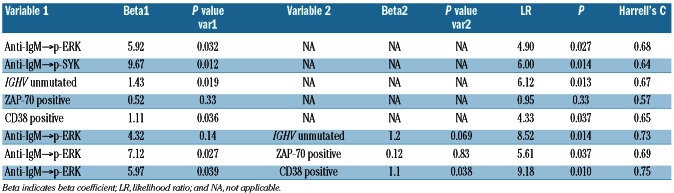
Application of the cutpoint determined in test study 1 for the Uu metric to the independent group from test study 2 demonstrated a significant difference in TTFT between samples from higher and lower anti-IgM-pERK responders, as determined by the log rank test for Kaplan-Meier estimates of TTFT (P=0.003) (Figure 3D).
Discussion
This study shows that BCR signaling responsiveness, characterized using SCNP, is of clinical relevance as it is associated with TTFT in unselected cohorts of patients and specifically in patients with early stage disease. Although several biological parameters enable classification of early stage B-CLL patients into risk groups, predicting which patients will progress (and require treatment) remains a significant clinical challenge. We propose that dynamic properties of BCR signaling measured by SCNP provide information that is independent of standard prognostic markers in predicting disease progression risk. Our findings also underscore the robustness of the biology measured using SCNP across multiple laboratories, with different protocols and three distinct populations of patients. These data may form the basis for future studies aimed at investigating the clinical value of BCR signaling profiles in predicting response to therapy and identifying deregulated signaling pathways as therapeutic targets in B-CLL.
The first observation from the exploratory study is the existence of profound quantitative differences in BCR signaling responsiveness, as measured by SCNP, between the subsets of B-CLL samples defined by IGHV mutational status, which confirms and extends previous findings7-9,27-28 and supports the notion that BCR functional activity is involved in B-CLL pathogenesis and disease course. Interestingly, SCNP data also reveal different signaling network connectivity in the two prognostic subsets. Specifically, in the subset of B-CLL with mutated IGHV, responses of SYK, NF-κB and ERK appear to be highly correlated, thus suggesting that these proteins belong to the same sub-network. In contrast, response of NF-κB appears to be unrelated with those of p-SYK and p-ERK in the UM B-CLL subset, thus suggesting that the NF-κB pathway activated by BCR is disjointed from SYK and ERK phosphorylation. Of note, p-SYK and p-ERK responses were inversely correlated in the UM B-CLL subset, indicating that ERK activation may be independent of SYK and that signaling pathways involving either SYK or ERK may be alternatively activated. In B cells, ERK can be activated via the PLCγ2 pathway or by RAS and both these pathways can be activated either by SYK or independently of it.29,30 We can, therefore, hypothesize that ERK pathways disjointed from SYK are prevalently activated in UM B-CLL cells, as opposed to the M B-CLL connectivity that resembles the normal B-cell one. Although this speculation deserves further investigations, it is reminiscent of recent data on miRNA networks in B-CLL showing the existence of independent leukemic miRNA subnetworks separated and disjointed from the main network.31
Single-cell analysis of BCR signaling also reveals a bimodal distribution of p-SYK and p-NF-κB in a small fraction of samples, both in unmodulated and modulated conditions. The nature of such a distribution is unknown but the positive cells may represent genetic or epigenetic subclones or cell states.
A large fraction of B-CLL cells showed constitutive phosphorylation of SYK and NF-κB, whereas basal phosphorylation of ERK was observed in 16 of 27 patients (59%) (data not shown), in accordance with Muzio et al., who reported constitutive phosphorylation of ERK1/2 in about half CLL patients' samples.32 Overall, the constitutive activity of SYK, NF-κB and ERK suggests that tonic, antigen-independent BCR signaling may have a role in B-CLL pathophysiological mechanisms. Consistently, it has been recently reported that cell-autonomous antigen-independent signaling is a crucial pathogenic mechanism in B-CLL.33 However, lack of association between tonic BCR signaling and prognostic parameters suggests that it is not clinically relevant. In contrast, quantification of BCR-induced signaling allows us to distinguish two main groups of B-CLL patients with different biological and clinical characteristics. Clustering patients based on BCR signaling profiles provides independent information that is at least as powerful in discriminating different disease courses as IGHV mutational status, the latter being the most robust prognostic marker in B-CLL so far (Table 1 and Online Supplementary Figure S4).
Specifically, a higher responsiveness to anti-IgM modulation in B-CLL was associated with shorter TTFT, even in early stage disease. Importantly, although anti-IgM→p-SYK was also associated with shorter TTFT in the exploratory study and test study 2, only the association of anti-IgM→p-ERK with clinical outcome observed in the exploratory study was independently confirmed in the test studies. In spite of the differences in outcome characteristics between the exploratory and test sample sets, the separation in TTFT in groups delineated by anti-IgM→p-ERK signaling was consistent (compare Figures 3A,B), suggesting that the functional characterization of patients' samples is robust. Whereas the surface expression of IgM is necessary for downstream anti-IgM induced signaling, analysis of signaling, as opposed to measuring surface receptor expression, captures the behavior of other components of the BCR pathway, supporting the direct functional interrogation of diseased cells (Online Supplementary Figure S6).
Differential effects of BCR ligation on p-ERK phosphorylation in unmutated versus mutated B-CLL clones has been previously described using different methodological approaches.7-9,27 The data of the present studies confirm and extend those results. Furthermore, they show that p-ERK is differently connected to the other nodes of the BCR signaling network in unmutated and mutated B-CLL subsets - a result that could only be obtained using a system-based approach. Most importantly, quantification of p-ERK by a SCNP assay allowed the use of the readout values to develop a mathematical model for prediction of B-CLL progression in early stage patients and to validate this model in independent sets of B-CLL patients. Modeling analysis showed that increased p-ERK signaling in response to anti-IgM had a higher prognostic power than CD38 (in the exploratory study) and ZAP-70 positivity (in the test studies). In all studies, however, the prognostic impact of the IGHV mutational status was higher than that of the other prognostic parameters, including p-ERK signaling. Methods to identify IGHV mutations are, however, not widely available in clinical practice. SCNP analysis of p-ERK response is a sensitive and robust method that might be readily available in general laboratories. Furthermore, the use of flow cytometry offers advantages for analyzing p-ERK selectively in B-CLL cell subpopulations. Taken together, these findings indicate that BCR modulated signaling provides information that is independent of currently used prognostic markers.
The present report suggests a prominent role of ERK activation induced by BCR modulation in B-CLL physiopathology. Accordingly, it has been recently shown that MEK inhibition blocks phosphorylation of the pro-apoptotic BCL2 family protein BIM and reduces pro-survival effects induced in B-CLL by co-culture systems,10 thus suggesting that MEK/ERK signaling functions as a survival pathway in B-CLL. Furthermore, the MEK/ERK pathway is required for B-CLL CpG-induced cell cycle progression.27-28 Interestingly, ERK phosphorylation has been detected in vivo specifically within the proliferative centers of B-CLL, sites of leukemic proliferation and encounter with antigen.27 Aberrant regulation of the ERK pathways contributes to the pathogenesis of cancer and much attention has been focused on developing inhibitors of the ERK signaling pathway and its upstream activators in many types of cancer,34 including B-CLL, in which pharmacological inhibition of the MEK/ERK pathways may be an attractive therapeutic choice, especially in progressive disease.
In summary, this report demonstrates the utility of the SCNP assay for identification and independent verification of BCR signaling profiles associated with the prognosis of B-CLL. Specifically, increased p-ERK signaling in response to anti-IgM was shown to be, by itself, a significant, independent predictor of shorter TTFT. The technical repeatability observed in the bridging study reflects the robustness of the biology measured and supports the potential for development of a SCNP-based prognostic/predictive test that will be useful in the daily management of patients with CLL.
Supplementary Material
Acknowledgments
The authors would like to thank all patients who donated samples for this study.
Funding: This study was funded by: Regione Veneto, Ricerca Sanitaria Finalizzata; Fondazione G. Berlucchi per la Ricerca sul Cancro; Fondazione Cassa di Risparmio di Verona, Vicenza, Belluno e Ancona and Associazione Italiana Ricerca sul Cancro (AIRC) (grant #6599); Fondazione Cassa di Risparmio di Verona, Vicenza, Belluno, e Ancona (grant #2008.14.44). This work was also supported in part by research funding from Nodality Inc. to AC, EE, J P, JC, REH, and JRW and in part by NIH grant RO1 CA81554 to NC.
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures: Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Wierda WG, O'Brien S, Wang X, Faderl S, Ferrajoli A, Do KA, et al. Prognostic nomogram and index for overall survival in previously untreated patients with chronic lymphocytic leukemia. Blood. 2007;109(11):4679-85 [DOI] [PubMed] [Google Scholar]
- 2.Shanafelt TD, Jenkins G, Call TG, Zent CS, Slager S, Bowen DA, et al. Validation of a new prognostic index for patients with chronic lymphocytic leukemia. Cancer. 2009;115(2):363-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wierda WG, O'Brien S, Wang X, Faderl S, Ferrajoli A, Do KA, et al. Multivariable model for time to first treatment in patients with chronic lymphocytic leukemia. J Clin Oncol. 2011;29(31):4088-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cramer P, Hallek M. Prognostic factors in chronic lymphocytic leukemia-what do we need to know? Nat Rev Clin Oncol.2011;8(1):38-47 [DOI] [PubMed] [Google Scholar]
- 5.Shanafelt TD. Predicting clinical outcome in CLL: how and why. Hematology Am Soc Hematol Educ Program. 2009;2009(1): 421-9 [DOI] [PubMed] [Google Scholar]
- 6.Zenz T, Mertens D, Küppers R, Döhner H, Stilgenbauer S. From pathogenesis to treatment of chronic lymphocytic leukaemia. Nat Rev Cancer. 2010;10(1):37-50 [DOI] [PubMed] [Google Scholar]
- 7.Scupoli MT, Pizzolo G. Signaling pathways activated by the B-cell receptor in chronic lymphocytic leukemia. Expert Rev Hematol. 2012;5(3):341-8 [DOI] [PubMed] [Google Scholar]
- 8.Stevenson FK, Krysov S, Davies AJ, Steele AJ, Packham G. B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2011;118(16):4313-20 [DOI] [PubMed] [Google Scholar]
- 9.Le Roy C, Deglesne PA, Chevallier N, Beitar T, Eclache V, Quettier M, et al. The degree of BCR and NFAT activation predicts clinical outcomes in chronic lymphocytic leukemia. Blood. 2012;120(2):356-65 [DOI] [PubMed] [Google Scholar]
- 10.Paterson A, Mockridge CI, Adams JE, Krysov S, Potter KN, Duncombe AS, et al. Mechanisms and clinical significance of BIM phosphorylation in chronic lymphocytic leukemia. Blood. 2012;119(7):1726-36 [DOI] [PubMed] [Google Scholar]
- 11.Damle RN, Temburni S, Banapour T, Paul S, Mongini PK, Allen SL, et al. T-cell independent, B-cell receptor-mediated induction of telomerase activity differs among IGHV mutation-based subgroups of chronic lymphocytic leukemia patients. Blood. 2012;120(12):2438-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gobessi S, Laurenti L, Longo PG, Carsetti L, Berno V, Sica S, et al. Inhibition of constitutive and BCR-induced Syk activation downregulates Mcl-1 and induces apoptosis in chronic lymphocytic leukemia B cells. Leukemia. 2009;23(4):686-97 [DOI] [PubMed] [Google Scholar]
- 13.Burger JA. Inhibiting B-cell receptor signaling pathways in chronic lymphocytic leukemia. Curr Hematol Malig Rep. 2012;7(1):26-33 [DOI] [PubMed] [Google Scholar]
- 14.Irish JM, Hovland R, Krutzik PO, Perez OD, Bruserud Ø, Gjertsen BT, et al. Single cell profiling of potentiated phospho-protein networks in cancer cells. Cell. 2004;118(2):217-28 [DOI] [PubMed] [Google Scholar]
- 15.Irish JM, Kotecha N, Nolan G P. Mapping normal and cancer cell signalling networks: towards single-cell proteomics. Nat Rev Cancer. 2006;6(2):146-55 [DOI] [PubMed] [Google Scholar]
- 16.Covey TM, Cesano A. Modulated multi-parametric phosphoflow cytometry in hematological malignancies: technology and clinical applications. Best Pract Res Clin Haematol. 2010;23(3):319-31 [DOI] [PubMed] [Google Scholar]
- 17.Kornblau SM, Minden MD, Rosen DB, Putta S, Cohen A, Covey T, et al. Dynamic single-cell network profiles in acute myelogenous leukemia are associated with patient response to standard induction therapy. Clin Cancer Res. 2010;16(14): 3721-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen DB, Minden MD, Kornblau SM, Cohen A, Gayko U, Putta S, et al. Functional characterization of FLT3 receptor signaling deregulation in acute myeloid leukemia by single cell network profiling (SCNP). PLoS One. 2010;5(10):e13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longo DM, Louie B, Putta S, Evensen E, Ptacek J, Cordeiro J, et al. Single-cell network profiling of peripheral blood mononuclear cells from healthy donors reveals age- and race-associated differences in immune signaling pathway activation. J Immunol. 2012;188(4):1717-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Covey TM, Cesano A, Parkinson DR. Single-cell network profiling (SCNP) by flow cytometry in autoimmune disease. Autoimmunity. 2010;43(7):550-9 [DOI] [PubMed] [Google Scholar]
- 21.Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O'Brien S, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87(12):4990-7 [PubMed] [Google Scholar]
- 22.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zanotti R, Ambrosetti A, Lestani M, Ghia P, Pattaro C, Remo A, et al. ZAP-70 expression, as detected by immunohistochemistry on bone marrow biopsies from early-phase CLL patients, is a strong adverse prognostic factor. Leukemia. 2007;21(1):102-9 [DOI] [PubMed] [Google Scholar]
- 24.Cesano A, Rosen DB, O'Meara P, Putta S, Gayko U, Spellmeyer DC, et al. Functional pathway analysis in acute myeloid leukemia using single cell network profiling (SCNP) assay: effect of specimen source (bone marrow or peripheral blood) on assay readouts. Cytometry B Clin Cytom. 2012;82(3):158-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361-87 [DOI] [PubMed] [Google Scholar]
- 26.R Development Core Team R: A language and environment for statistical computing. http://www.R-project.org Accessed January 31, 2012
- 27.Krysov S, Dias S, Paterson A, Mockridge CI, Potter KN, Smith KA, et al. Surface IgM stimulation induces MEK1/2-dependent MYC expression in chronic lymphocytic leukemia cells. Blood. 2012;119(1):170-9 [DOI] [PubMed] [Google Scholar]
- 28.Longo PG, Laurenti L, Gobessi S, Petlickovski A, Pelosi M, Chiusolo P, et al. The Akt signaling pathway determines the different proliferative capacity of chronic lymphocytic leukemia B-cells from patients with progressive and stable disease. Leukemia. 2007;21(1):110-20 [DOI] [PubMed] [Google Scholar]
- 29.Kurosaki T. Regulation of B-cell signal transduction by adaptor proteins. Nat Rev Immunol. 2002;2(5):354-63 [DOI] [PubMed] [Google Scholar]
- 30.Nagaoka H, Takahashi Y, Hayashi R, Nakamura T, Ishii K, Matsuda J, et al. Ras mediates effector pathways responsible for pre-B cell survival, which is essential for the developmental progression to the late pre-B cell stage. J Exp Med. 2000;192(2):171-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volinia S, Galasso M, Costinean S, Tagliavini L, Gamberoni G, Drusco A, et al. Reprogramming of miRNA networks in cancer and leukemia. Genome Res. 2010;20(5):589-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muzio M, Apollonio B, Scielzo C, Frenquelli M, Vandoni I, Boussiotis V, et al. Constitutive activation of distinct BCR-signaling pathways in a subset of CLL patients: a molecular signature of anergy. Blood. 2008;112(1):188-95 [DOI] [PubMed] [Google Scholar]
- 33.Dühren-von Minden M, Ubelhart R, Schneider D, Wossning T, Bach MP, Buchner M, et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature. 2012;489(7415):309-12 [DOI] [PubMed] [Google Scholar]
- 34.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26(22): 3291-310 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.