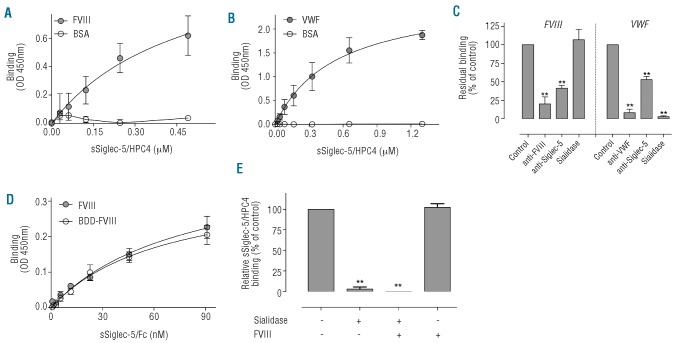
Figure 1.
Binding of soluble Siglec-5 to immobilized FVIII or VWF. (A and B) sSiglec-5/HPC4 (0-1.3 mM) was incubated with highly purified plasma-derived FVIII (A, closed circles), VWF (B, closed circles) or BSA (open circles) immobilized onto microtiter wells (all 2.5 μg/mL) in 0.15 M NaCl, 5 mM CaCl2, 0.1% Tween-20, 0.5% PVP, 25 mM Tris-HCl (pH 7.4). After 3 h of incubation, bound sSiglec-5/HPC4 was probed with peroxidase-labeled antibody HPC4 for 2 h at 37°C and detected by peroxidase hydrolysis of TMB. Presented is the absorbance at 450 nm versus sSiglec-5/HPC4 concentration. (C) Binding of sSiglec-5/HPC4 to immobilized FVIII or VWF was performed in the absence or presence of anti-FVIII, anti-VWF or anti-Siglec-5 antibodies. Alternatively, immobilized FVIII or VWF was incubated in the absence or presence of sialidase (0.1 U/mL) for 16 h at 37°C prior to incubation with Siglec-5/HPC4. Residual sSiglec-5/HPC4 binding compared to the binding in the absence of antibodies or sialidase is shown. (D) Plasma-derived full-length FVIII (closed circles) or recombinant BDD-FVIII (open circles) were adsorbed onto microtiter wells coated with monoclonal anti-FVIII antibody D4H1, and subsequently incubated with sSiglec-5/Fc (0-90 nM). Bound sSiglec-5/Fc was probed with peroxidase-labeled anti-human Fc antibody and detected via peroxidase hydrolysis of TMB. Shown is the absorbance at 450 nm versus sSiglec-5/Fc concentration. (E) VWF (2.5 μg/mL) was immobilized and incubated in the absence or presence of sialidase (0.1 U/mL) for 16 h at 37°C. Immobilized VWF was then incubated in the absence or presence of FVIII (5 μg/mlL for 1 h at 37°C. Finally, the wells were incubated with sSiglec-5/HPC4 (0.33 μM) and bound sSiglec-5/HPC4 was probed as described for panels A and B. The data present the mean±SEM of 3-5 independent experiments. The drawn lines (A,B and D) were obtained by fitting the data to an equation describing the binding of soluble Siglec-5 to a single class of binding sites, and were used to calculate half-maximal binding. **P<0.001.