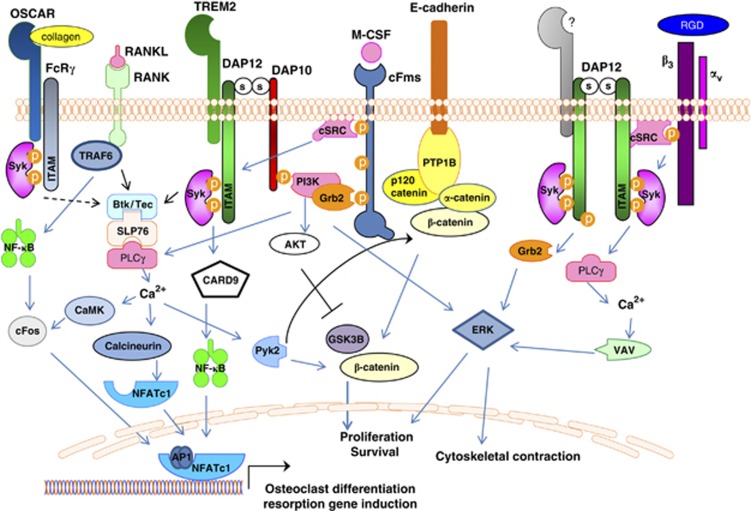
Figure 2. Synergy of osteoclast signaling pathways.
Osteoclast differentiation is induced by integration of M-CSF, RANKL and ITAM signaling. DAP12 co-stimulates RANK signaling by Syk-induced activation of BTK and Tec kinases, as well as PI3K activation leading to PLCγ and calcium oscillations. Ca2+-stimulated calcineurin dephosphorylates NFATc1, enabling nuclear translocation of NFATc1. DAP12-induced Ca2+ drives CaMKIV (calcium/calmodulin-dependent protein kinase type IV), which further activates RANK-induced cFos to translocate to the nucleus and form AP-1 dimers that work with NFATc1 to activate osteoclast-specific genes. Binding of M-CSF to its receptor, cFms, leads to recruitment of cSrc that phosphorylates the DAP12 ITAM, inducing Syk binding and activation. Syk subsequently activates PI3K, likely recruited to the membrane predominately via DAP10, leading to activation of PLCγ, VAV and ERK, culminating in actin cytoskeletal rearrangements. Activation of DAP12 by c-Fms-recruited cSrc induces Ca2+-stimulated Pyk2 release of β-catenin from an E-cadherin complex, thus allowing the release and nuclear accumulation of β-catenin to drive osteoclast cell proliferation and survival. DAP12-induced AKT activation further facilitates this process by inhibiting GSK3B. Integrin αvβ3 recruits cSrc to activate DAP12 leading to activation of ERK, PLCγ and VAV to mediated cytoskeleton rearrangement. OSCAR, an FcRγ-associated receptor, acts as a collagen receptor and can provide necessary ITAM co-stimulation of RANK in the absence of DAP12.