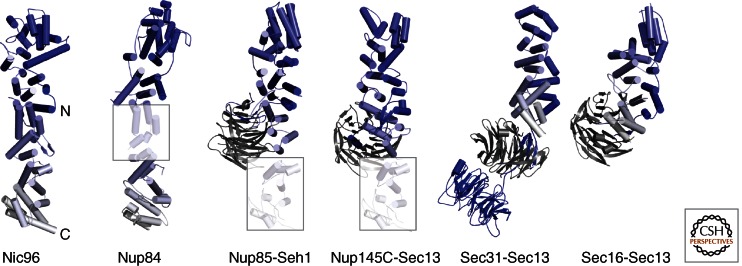
Figure 2.
The ancestral coatomer element ACE1. Four nucleoporins (Nic96, Nup84, Nup85, Nup145C) share a specific α-helical stack domain with Sec31 and Sec16 of the COPII vesicle system. The six proteins are aligned such that ACE1, shown in a blue-white color gradient from the amino to the carboxyl terminus, is oriented similarly. ACE1 is characterized by a fold-back architecture, which is highly unusual for α-helical stack domains. The 65-kDa ACE1 core has 28 α-helices. In Nup84, Nup85, and Nup145C, parts of the ACE1 domain are homology-modeled (shaded boxes) based on the Nic96 structure. Nup85, Nup145C, Sec31, and Sec16 bind the six-bladed, open β-propeller proteins Seh1 and Sec13 (gray), respectively, by inserting a seventh blade. The orientation relative to the ACE1 domain can vary. Sec31 contains an additional β-propeller at its amino terminus. ACE1 has not yet been found outside of the NPC and COPII, indicating a distinct evolutionary relationship.