Figure 2.
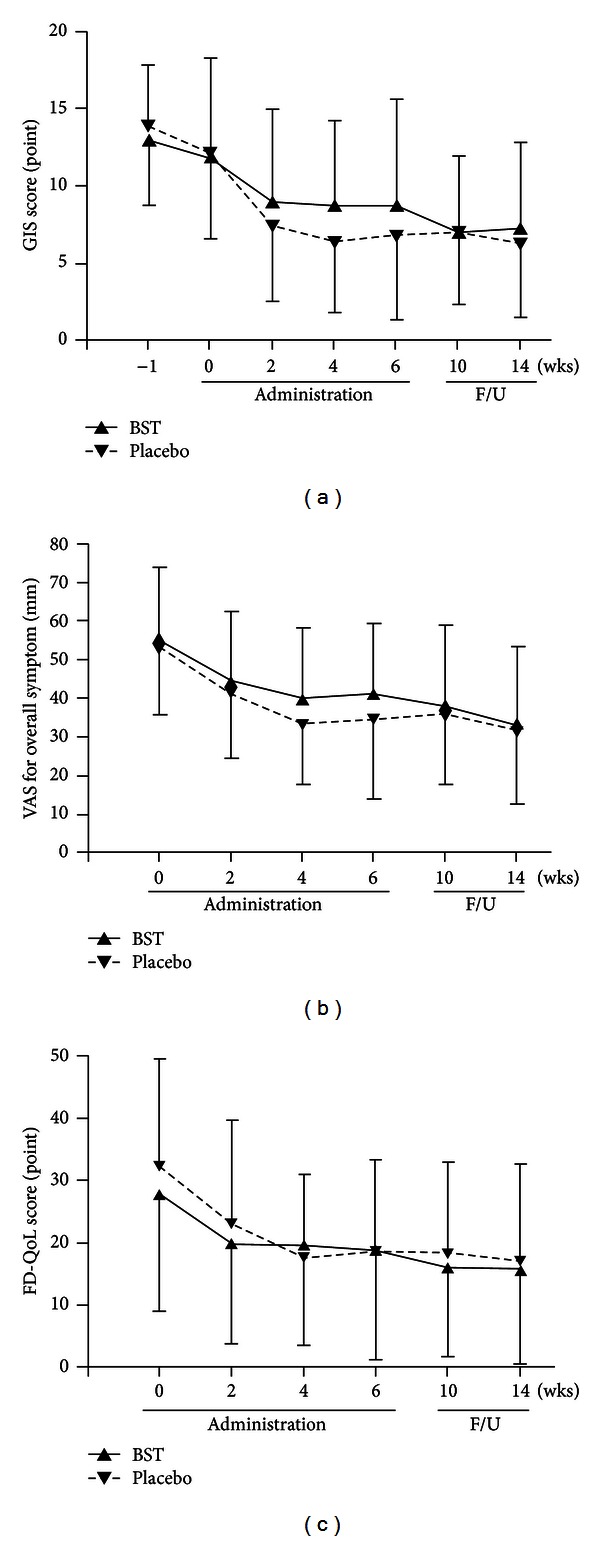
Changes in main outcomes of the trial. (a) Changes in the total scores of gastrointestinal symptom (GIS) scale between BST and placebo groups during the whole trial. (b) Changes in visual analogue scale (VAS) for overall symptoms between BST and placebo groups during the whole trial. (c) Changes in the total scores of functional dyspepsia-related quality of life (FD-QoL) between BST and placebo groups during the whole trial. BST: Banha-sasim-tang. F/U: follow-up period.
