Abstract
Host ecological traits may limit exposure to infectious disease, thereby generating the wide variation in disease incidence observed between host populations or species. The exclusion of disease by ecological traits may then allow selection to act against physiological defenses when they are costly to maintain in the absence of disease. This study investigates ecological resistance in the Silene-Microbotryum pathosystem. An estimated 80% of perennial Silene species host the anther-smut disease while no annuals harbor the disease in nature. Artificial inoculations of annual and perennial Silene plants, obtained from both natural and horticultural populations, demonstrate that the absence of disease in annuals is not explained by elevated physiological resistance. The annual habit is thus a powerful form of ecological defense against anther smut. Moreover, the higher susceptibility of annual species to anther smut relative to perennials supports the hypothesis of a loss of costly physiological resistance under ecological protection. The observation in annuals that physiological susceptibility is correlated with lower rates of flowering (i.e., lower fitness) suggests that variation in physiological resistance is costly in the absence of disease, even in a naїve Silene species. The absence of disease in natural populations of annuals combined with their high physiological susceptibility attest to the strength of host ecology in shaping the distribution of disease and to the dynamic nature of disease resistance.
Keywords: Annual, disease resistance, Microbotryum, perennial, Silene
Introduction
Members of the same or closely related species frequently exhibit great disparities in their incidence of infectious disease. Explanations for this variation in susceptibility between individuals or populations are manifold, ranging from fine-scale variation in immune traits (Graham et al. 2010; Telfer et al. 2010) and social interactions (Tinsley 1989; Ezenwa 2004; Hawley et al. 2006) to broad-scale variation in environmental factors (Pedersen and Grieves 2008; Duffy et al. 2012) and demography (Nunn et al. 2003; Altizer et al. 2007; Lindenfors et al. 2007). Most of these host traits influencing pathogen susceptibility can be divided into two broad categories: physiology and ecology. Physiological defenses are biochemical, immunological, and structural mechanisms that determine the proportion of individuals remaining healthy upon direct exposure to a pathogen. Alternatively, ecological defenses encompass behavioral, life-history, and demographic traits that limit exposure to a pathogen (Ouborg et al. 2000).
Ecological differences have been cited in a wide variety of taxa to explain disease distributions or to estimate risk from emerging infectious diseases (e.g., Pedersen et al. 2007; Jones et al. 2008; Duffy et al. 2012; Johnson et al. 2012; Swinfield et al. 2012). Examples include age (Lafferty 1993; Jokela and Lively 1995; Fredensborg and Poulin 2006) or sex-specific behaviors (Alexander 1989; Ezenwa 2004), as seen in the North American desert toad: females spend less time in water and therefore carry only a quarter of the number of parasitic aquatic trematodes as do males (Tinsley 1989). Prevention of disease by large-scale demographic factors has been reported in primates (Nunn et al. 2003; Altizer et al. 2007) and carnivores (Lindenfors et al. 2007). In both cases, fewer parasites are found in species exhibiting reduced population density and connectivity. Similarly, Plowright et al. (2011) demonstrate that variation between populations of flying fox species in these same factors influences the size and persistence of Hendra virus outbreaks, as well as its probability for emergence in humans. These examples highlight the disparity in disease distributions that can exist within and between species when strong ecological contrasts are present.
Hood et al. (2010) recently surveyed over 40,000 herbarium specimens for the presence of the disease anther smut. All specimens were collected from natural populations of plants in the genus Silene and allied genera of the family Caryophyllaceae. This study revealed that the fungal disease is entirely restricted to perennial species. The contrast between annuals and perennials remains even after controlling for phylogenetic relationships within the clade. These results agree with those of Thrall et al. (1993), who found fewer literature reports of anther smut on annual than perennial species. Both studies attribute the lack of anther smut in annuals to the particular life cycle of the pathogen.
This disease results from infection by pollinator-transmitted fungal pathogens in the genus Microbotryum. Infection leads to host sterilization by abortion of female structures and replacement of pollen with dark-colored fungal spores (Fig. 1). Disease transmission occurs primarily through spores vectored by pollinators from infected to healthy plants (Alexander and Maltby 1990). Spore germination, meiosis, and mating of haploid sporidial cells of opposite mating types occur on the host plant surface prior to infection (reviewed in Giraud et al. 2008). The pathogens are believed to lack any environmentally-resilient resting stages, thus requiring overwintering within the living host. It is this feature of anther smut that has been hypothesized to make annual plants, which do not overwinter, inviable hosts of anther-smut disease (Hood et al. 2010). Accordingly, these studies suggested that the annual habit can function in the Silene-Microbotryum system as an ecological defense, restricting the disease's distribution.
Figure 1.

Expression of anther-smut disease upon inoculation of the annual plant species Silene colorata with the fungal pathogen Microbotryum lychnidis-dioicae. The pollen has been replaced by dark-colored fungal spores.
Effects of other ecological traits have also been characterized in this system. For instance, the disease is absent from species in the Caryophyllaceae that have cleistogamous (i.e., “closed mating”) flowers, presumably due to lack of interaction with pollinators that vector the fungal spores (Thrall et al. 1993). Furthermore, despite exhibiting the perennial life history, Silene species threatened with extinction appear to have lost the disease, potentially due to their occurrence in small, fragmented populations where transmission is suppressed (Gibson et al. 2010). At the within-species level, Silene latifolia shows lower infection prevalence in genotypes with shortened or delayed flowering seasons (Alexander et al. 1993; Biere and Antonovics 1996).
The marked absence of disease on annual species is consistent with ecological resistance. The possibility remains, however, that the absence of anther smut in annual Caryophyllaceous species is instead due to strong physiological resistance, as prior studies on disease and life history were correlative (Thrall et al. 1993; Hood et al. 2010). The Silene-Microbotryum system is well suited to test the relative contributions of ecological and physiological resistance. Artificial inoculations of host plants with Microbotryum isolates allow direct, experimental estimation of physiological resistance to anther-smut disease. A genetic basis for resistance to anther smut has been demonstrated in multiple Silene species (Alexander 1989; Alexander et al. 1993; Cafuir et al. 2007). Moreover, it is one of the few natural systems where the costs of resistance have been quantified (Alexander 1989; Biere and Antonovics 1996).
In this study, we aim to directly measure physiological resistance of annuals to determine whether host ecological traits are responsible for their disease-free status. In addition, we assess the possibility that, in the presence of a strong ecological defense, natural selection may favor the loss of mechanisms of physiological resistance, which are often costly to maintain in the absence of disease (Alexander 1989; Simms 1992; Biere and Antonovics 1996). We would predict such selection to manifest as annual species exhibiting greater physiological susceptibility than perennial species. We estimate physiological resistance using experimental inoculations of annual and perennials in the genus Silene to compare infection rates upon direct exposure to Microbotryum pathogens. Moreover, we quantify the costs associated with variation in physiological resistance in one species of annual host. By addressing fundamental life-history differences as ecological determinants of disease risk, this study finds that host ecology governs pathogen distributions in nature and shapes the dynamics of host-pathogen coevolution.
Material and Methods
Model system
Fungal pathogens of the Microbotryum violaceum species complex (Basidiomycetes: Microbotryales) represent a suite of divergent, host-specific species (Le Gac et al. 2007; Lutz et al. 2008). Host species are primarily members of the Caryophyllaceae family and are particularly overrepresented in the Silene genus. Recently, Hood et al. (2010) estimated that approximately 80% of perennial species in the tribe Sileneae, within the Caryophyllaceae, are likely to host Microbotryum pathogens in nature. Infection causes anther-smut disease, of which the primary symptom is sterilization. Sterilization is often partial in the first season of infection, but anther smut is fully expressed in all flowers in subsequent years (Fig. 1). Effects upon host mortality are minimal, but the disease can impact population growth rates and persistence (Antonovics 2004).
Artificial inoculations
All plant species used in this study are members of the tribe Sileneae in the Caryophyllaceae (Oxelman et al. 2001). Sources of seeds are provided in Table S1. A total of eight annual species, represented by nine populations, and five perennial species, represented by eight populations, were inoculated. Annuals were identified by a review of published species descriptions in floras (see Hood et al. 2010). The included species represent at least three independent origins of the annual habit in the Silene genus (Oxelman et al. 2001; Rautenberg et al. 2012, B. Oxelman, unpubl. data). Perennial hosts used as controls are species known to maintain anther smut in the wild. They were therefore predicted to be susceptible to disease under artificial inoculation. Controls were intended to verify efficacy of the inoculation technique and to provide standard disease rates for comparison to those of annuals.
Seeds were surface-sterilized prior to germination, and upon emergence of cotyledons, seedlings were inoculated with four μL of Microbotryum according to Hood and Antonovics (2000). Two different inoculation treatments were applied to each set of seeds. This approach was taken to increase the probability of inoculating annual species with compatible pathogen strains. Unlike perennial Silene, annual species are not infected in nature and therefore do not have a native strain of Microbotryum. The first treatment (the ‘combined’ inoculum) comprised a pooled mixture of a1 and a2 haploid cells (sporidial cultures) from field collections of Microbotryum isolates infecting 13 different host species and representing at least nine different pathogen species (Table S2) (Le Gac et al. 2007). The second treatment (the ‘single’ inoculum) comprised a mix of a1 and a2 sporidia from a single pathogen species, which was selected from the previous list (Table S2) in an attempt to minimize the genetic distance of the pathogen's native host (i.e., the host-of-origin) and the host used as the target of artificial inoculations (see ‘Estimating phylogenetic constraints’ below).
Plants were grown in the greenhouse until flowering, at which time they were assessed for the presence of Microbotryum spores in their anthers. Flowers were collected prior to opening, when petals had extended further than the calyx teeth, or immediately upon opening to minimize contamination between inoculated plants. Diseased flowers were scored for the normality of disease expression, including whether anther filaments were fully elongated and the anther sacks had ruptured to reveal powdery and dehiscent fungal spores. Flowers of all diseased individuals were collected and stored under desiccation.
If examination under the stereo microscope was insufficient to determine disease status, the presence of Microbotryum was ascertained using PCR amplification. DNA was extracted from anthers by the Chelex method described in Bucheli et al. (2001). Standard PCR reactions were performed using Microbotryum-specific primers that amplify variable regions of the internal transcribed spacer (ITS) region of the nuclear rRNA genes described in Hood et al. (2010). Presence of infection was assigned upon visual examination of PCR products after separation in 1% agarose gels.
Estimating phylogenetic constraints
To investigate the potential influence of host specificity on pathogen success, the rates of infection and the normality of symptoms in the single inoculum treatment were tested for dependence upon the genetic distance between the inoculated annual species and the pathogen's perennial host-of-origin. Sequences for the internal transcribed spacer (ITS) region of the nuclear rRNA genes and the chloroplast rps16 intron were obtained from the NCBI database, from the online Sileneae database (http://www.sileneae.info), or directly from plants in the greenhouse. Greenhouse specimens used for DNA sequencing were preserved as herbarium sheets. DNA was extracted using the Plant DNeasy Kit (Qiagen, Hilden, Germany) following the manufacturer's protocol. The nuclear ITS1-4 and plastid intron rps16 regions were amplified from extracted plant DNA using standard PCR methods and primers given in White et al. (1990) and Popp and Oxelman (2007), respectively. Sequences were generated at the Molecular Genetics Core Facility at Penn State (http://med.psu.edu/web/core/molecular-genetics-services). GenBank (NCBI) accessions generated in this study are as follows: JX560218ITS and JX560215rps16 for S. cisplatensis, JX560219ITS and JX560216rps16 for S. colorata, JX560214rps16 for S. gallica, JX560220ITS and JX560217rps16 for S. germana, and JX560221ITS for S. macrodonta. Sequences for each locus were aligned using CLUSTAL-W implemented in MEGA4 (Tamura et al. 2007) and concatenated for analysis of genetic distances using the Kimura 2-parameter model with pairwise deletion.
Quantification of costs of physiological resistance
Physiological resistance to Microbotryum can be costly, as demonstrated by a negative association with surrogate fitness measures in natural perennial hosts (Biere and Antonovics 1996; Biere and Honders 1996). To evaluate whether costs of physiological resistance are also present in naïve annual species, five families of the annual species S. macrodonta were used. The families were produced by self-pollinating plants grown from field-collected seeds. For each family, two sets of 60 plants were assessed for infection following separate inoculations with two phylogenetically distant Microbotryum species: Microbotryum originating from S. latifolia and Microbotryum from Lychnis flos cuculi (N surviving plants among families: 36 ± 4). For each family, two additional sets of plants were grown for two months without pathogen exposure to assess the proportion of plants that flowered as a measure of fitness (N among families: 15 ± 2).
Statistical analysis
To assess differences among treatments in rates of infection and the normality of symptom development, statistical comparisons were made using generalized linear models in SPSS version 16 (SPSS Inc., Chicago, IL). A binomial logit function was assumed, and the model included the effect of host lifespan (annual vs. perennial), inoculum type (combined vs. single), and the interactions between these terms in predicting the dependent variable of the proportions of plants that became diseased. The analysis was performed on the complete dataset, including entries for each population nested within species (i.e., for S. latifolia and S. noctiflora) as well as with the plants from separate populations pooled within species. Regression analyses incorporating host phylogenetic distances were performed in SPSS using a weighted least-squares linear regression model, in which the frequency (p) of disease or of normal anther-smut symptoms was weighted by the inverse of its variance, where var(p) = p(1−p)/n. Data for perennial hosts, which received their endemic pathogen in the single inoculum treatment, were excluded from this analysis. Variations in infection rates and flowering patterns were analyzed with a full factorial general linear model in SPSS, with infection rate as the dependent variable and pathogen type and proportion flowering as the fixed factor and covariate, respectively. Proportional data were arcsine-square root transformed for analysis.
Results
Four of five perennial species were successfully infected with Microbotryum. For three of these infected species, average infection rates across both inoculum treatments were greater than 60% (Table 1; Fig. 2). Seven of eight annual species were successfully infected with Microbotryum. For six of these infected species, average infection rates across both inoculation treatments were approximately 60% or greater. For two annual species, S. cisplatensis and S. macrodonta, average infection rates exceeded 90%.
Table 1.
Proportion of annual and perennial host species diseased and displaying normal symptoms following inoculation with Microbotryum under single and combined treatments
| Proportion diseased (N) | Proportion normal disease expression (N) | ||||
|---|---|---|---|---|---|
| Combined inoculum | Single inoculum | Combined inoculum | Single inoculum | Host source for single inoculum | |
| Annual species | |||||
| Atocion armeria | 0.87 (15) | 0.89 (19) | 0.93 (13) | 0.89 (17) | A. rupestre |
| Silene cisplatensis | 1.00 (11) | 1.00 (10) | 0.00 (11) | 0.00 (10) | V. alpina |
| Silene colorata | 0.27 (11) | 0.00 (14) | 0.67 (3) | S. acaulis | |
| Silene conica | 1.00 (7) | 0.43 (7) | 0.71 (7) | 0.86 (3) | S. latifolia |
| Silene gallica | 0.00 (14) | 0.00 (14) | S. acaulis | ||
| Silene germana | 0.42 (12) | 1.00 (14) | 0.00 (5) | 0.00 (14) | S. latifolia |
| Silene macrodonta | 1.00 (28) | 0.88 (42) | 1.00 (28) | 0.95 (37) | S. latifolia |
| Silene noctiflora | 0.42 (19) | 0.66 (53) | 0.13 (8) | 0.40 (35) | S. latifolia |
| Silene noctiflora | 0.30 (30) | 0.92 (24) | 0.13 (9) | 0.73 (22) | S. latifolia |
| Perennial species | |||||
| Atocion rupestre | 0.80 (10) | 0.73 (11) | 0.80 (8) | 1.00 (8) | |
| Silene italica | 0.20 (5) | 1.00 (7) | 1.00 (1) | 1.00 (7) | |
| Silene latifolia | 0.08 (29) | 0.81 (36) | 1.00 (12) | 1.00 (36) | |
| Silene latifolia | 0.38 (19) | 0.85 (36) | 1.00 (5) | 1.00 (33) | |
| Silene latifolia | 0.26 (24) | 0.92 (21) | 1.00 (2) | 1.00 (17) | |
| Silene latifolia | 0.41 (13) | 1.00 (26) | 1.00 (5) | 1.00 (22) | |
| Silene uniflora | 0.00 (47) | 0.00 (47) | |||
| Silene vulgaris | 0.17 (36) | 0.04 (26) | 1.00 (6) | 1.00 (1) | |
Each proportion is followed by its sample size in parentheses. The host species from which the single inoculum was derived is given for each annual species. Each perennial species was singly inoculated with its native pathogen. The pathogen species associated with each host species are provided in Table S2.
Figure 2.
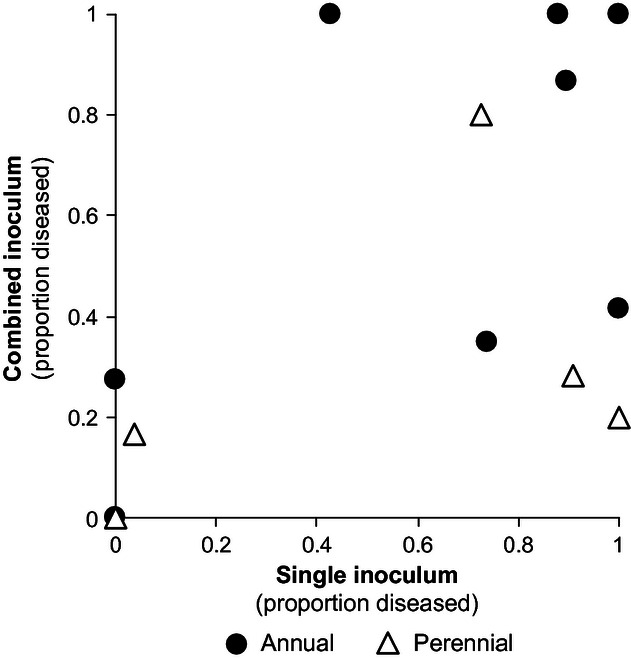
The relationship between the proportions of annual and perennial species infected as the result of inoculation with either a single or a combined mixture of Microbotryum species. Proportion of diseased individuals is given for five perennial (open triangles) and eight annual (closed circles) Silene species. Plants from multiple populations were pooled within species as indicated in the Material and Methods section, and sample size for the calculated proportions are shown in Table 1.
When plant populations were pooled according to species, all model parameters significantly affected infection rates, including effects of annual versus perennial lifespan (Wald's χ2 = 5.3, df = 1, P = 0.021), inoculum treatments of a single Microbotryum species versus the combined mixture of multiple Microbotryum species (Wald's χ2 = 53.49, df = 1, P < 0.001), and the interaction between these terms (Wald's χ2 = 11.82, df = 1, P = 0.001). Overall, annual species became diseased at a higher rate than perennial species, and the combined inoculum treatment resulted in higher rates of infection. Additionally, annual species were more likely to become diseased from the combined inoculum treatment whereas perennial species were more likely to become diseased from the single inoculum treatment (Fig. 2). The analysis performed with the inclusion of separate populations within species did not alter the level of statistical significance for any model parameter.
Disease expression was not uniform across treatments. Infections with normal disease expression were considered to be those in which anthers of the infected host plant were fully developed and bore dehiscent spore masses. Perennial hosts displayed normal infection symptoms in 99.0% of diseased plants (N = 163), whereas annual species exhibited normal symptoms in only 59.6% of diseased plants (N = 222) (Table 1). These data were insufficient to run the full linear regression model to test for the interaction term (lifespan x inoculum type). The reduced model excluding the interaction term provided a highly significant main effect for lifespan (Wald's χ2 = 48.67, df = 1, P < 0.001), but no significant effect of inoculum type (Wald's χ2 = 0.86, df = 1, P = 0.355). The analysis performed with the inclusion of separate populations within species did not alter the level of statistical significance for any model parameter, noting that the separate populations within species had the same proportions of plants showing normal disease expression (Table 1).
Excluding data on perennial species, which received their endemic pathogens as inoculum, there was no significant evidence that specialization by the pathogen influenced infection rates among annual species, as would be determined by a negative relationship between infection rate and the genetic distance between the inoculated annual species and the pathogens' perennial hosts-of-origin (β = −0.392, P = 0.337). However, the genetic distance effect was significant in determining the proportion of infected plants with normal symptom development (β = −0.855, P = 0.030), with distant cross-inoculations more frequently resulting in underdeveloped anthers and/or nondehiscent spore masses.
Quantification of the costs of resistance in greenhouse-generated families of the annual species S. macrodonta identified a trade-off between resistance, when inoculated with Microbotryum, and flowering rate when uninoculated. A family's proportion of individuals that flowered among uninoculated plants was a significant positive predictor of infection rates among inoculated plants (transformed data, effect of flowering rate F(2, 1) = 17.07, P = 0.007) (Fig. 3). Effects of pathogen type and their interaction were not significant.
Figure 3.
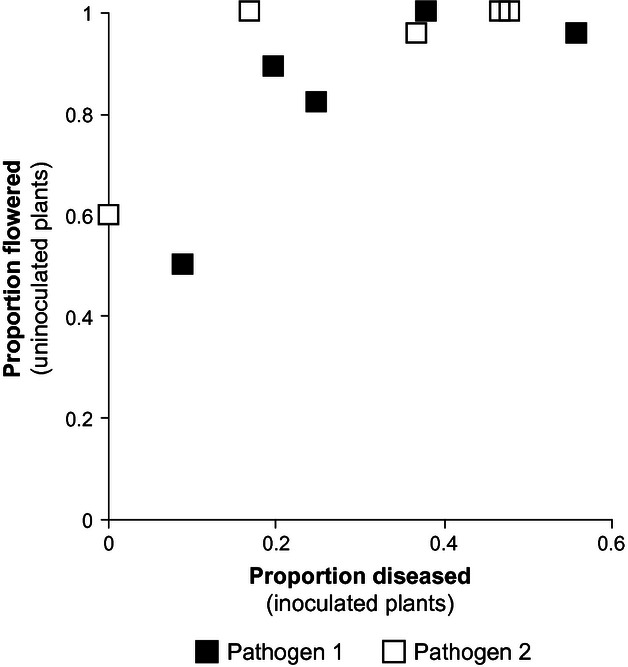
The relationship between the proportion infected by Microbotryum and flowering rate among families of the annual species Silene macrodonta. Infection rates were replicated across two pathogen species: Pathogen 1 = Microbotryum from Lychnis flos cuculi (closed squares) and Pathogen 2 = Microbotryum from Silene latifolia (open squares). Plant families were generated in the greenhouse. Infection rate was obtained using artificial inoculations while flowering rate was measured for uninoculated plants. Sample sizes for the calculated proportions are shown in Table S3.
Discussion
Anther-smut disease exclusively infects perennial plant species in nature, suggesting that an incompatibility with this fungal pathogen's disease cycle confers ecological protection in annual plant populations (Thrall et al. 1993; Hood et al. 2010). In this study, we compared the susceptibility to infection of annual and perennial Silene hosts in order to assess whether host ecology is a valid explanation for the absence of disease observed in annual species. We also investigated the potential loss of physiological resistance in annual hosts, as would be consistent with natural selection against costly physiological mechanisms of defense when there is strong ecological protection (Simms 1992). Our results indeed provide empirical support for the hypothesis that the annual habit serves as a form of ecological resistance against anther-smut disease. Moreover, they are consistent with a loss of physiological resistance under ecological resistance: annuals show lower physiological resistance than perennial species, and we demonstrate costs of physiological resistance in naïve annuals.
Attributing the absence of disease in annual populations to ecological rather than physiological resistance requires that artificial inoculations produce infection rates on annuals that are statistically as great as or greater than on perennials hosts. We provide evidence here that annual species become diseased at a significantly higher rate than perennial species under artificial inoculation. As verification of the artificial inoculation protocol used here, we find that infection rates on perennial species are comparable to those measured in prior studies using similar techniques (Biere and Honders 1996; Antonovics et al. 2002; Hood 2003; Gold et al. 2009). Moreover, the variation in infection rates observed between species is unlikely to be a technical artifact, as previous greenhouse and field experiments have shown that it represents heritable variation in physiological resistance (Alexander et al. 1993; Biere and Honders 1996). Therefore, our finding of high rates of infection upon direct exposure to Microbotryum supports the hypothesis that ecological factors are the primary determinants preventing disease persistence in natural populations of annual Silene species (Hood et al. 2010).
The annual habit of the Silene hosts addressed here contributes to disease protection in a manner similar to other life-history traits, such as lifespan and timing of reproductive maturity. Many studies implicate changes in life-history traits as adaptive responses to parasite pressure. If the threat of parasitism accumulates with age and parasitism is associated with a reduction in fecundity, life-history theory predicts a shift toward earlier host reproduction in the presence of parasites (Minchella 1985; Hochberg et al. 1992; Agnew et al. 2000). Similarly, earlier reproduction or shortened lifespan can, under certain conditions, reduce the need for an individual to invest in costly resistance mechanisms, such as innate immunity (Minchella 1985; Miller et al. 2007; Lee et al. 2008). Empirical studies have supported these predictions (as reviewed in Agnew et al. 2000). Agnew et al. (1999) observed accelerated development and earlier reproduction in infected female mosquitoes. Three separate studies of gastropods show a significant negative correlation between average size at reproductive maturity and trematode infection prevalence, consistent with reproduction at an earlier age in parasitized populations (Lafferty 1993; Jokela and Lively 1995; Fredensborg and Poulin 2006). Our finding that the annual habit is a form of ecological resistance against anther-smut disease (Thrall et al. 1993; Hood et al. 2010) further supports these previous studies in demonstrating that life-history traits serve as powerful defenses against parasitism.
A key distinction between the annual habit and the life-history shifts discussed in these previous studies must, however, be made: it cannot be inferred that the annual habit evolved as an adaptive response to parasitism. We show here that annual species are susceptible to infection with anther smut. Therefore, individual plants in a diseased population may not experience a gain in fitness by reducing lifespan. Rather, the primary benefit of the annual habit seems to manifest at the group level, with populations of annuals being incapable of maintaining the pathogen across years (Hood et al. 2010). Further studies are required to determine whether a group selection argument is required for disease to drive a shift from a perennial to annual life history. An alternative scenario is that the annual habit has evolved in response to unrelated selection pressures and has subsequently influenced disease transmission and infection. Indeed, transitions between the annual and perennial habit depend upon a complex balance of investment in reproduction and growth so as to optimize lifetime reproductive fitness. This balance is altered by numerous external factors, including environmental stability and, perhaps, disease (Cole 1954; Gadgil and Bossert 1970; Charnov and Schaffer 1973; Gaines et al. 1974; Hart 1977).
Regardless of the evolutionary history of the host trait, a mechanism that excludes a common and damaging disease can be expected to leave a distinct mark upon the patterns of natural selection in the host. We show here that the strong ecological resistance of annual Silene hosts is associated with lower physiological resistance: upon direct exposure, annuals became infected by Microbotryum more frequently than did perennial hosts. This result suggests that costly physiological resistance is lost under powerful ecological resistance. Indeed, we find evidence for such costs of physiological resistance in annuals: variation in susceptibility among families of the annual S. macrodonta was correlated with flowering patterns in a manner similar to reported costs of resistance in perennial hosts. Biere and Antonovics (1996) showed that resistant S. latifolia genotypes suffer a fitness cost relative to more susceptible genotypes in the absence of disease. Alexander et al. (1996) found that late-flowering genotypes of S. latifolia became less diseased in the field, and Biere and Honders (1996) suggested that physiological resistance was also associated, albeit indirectly, with delayed flowering. Our study suggests that a similar correlation between resistance and flowering may exist even in potential host species that have not experienced the disease historically. Preexisting variation in resistance can have major implications for the emergence of new diseases. Accordingly, studies on a broader range of annual plants are needed to assess the generality of this variation.
It is probable that our estimation of susceptibility here even underestimates the extent to which physiological resistance is absent in annual species. Annual species are disease free in nature and so were inoculated with nonendemic pathogens. In multiple studies, such novel host-pathogen combinations perform relatively poorly, resulting in lower infection rates than inoculations of Microbotryum strains on their hosts-of-origin (Sloan et al. 2008; de Vienne et al. 2009). A significant negative correlation was lacking between infection rate and genetic distance from the hosts-of-origin to inoculated annual species; this may reflect a lack of statistical power or an insufficient range of host genetic distances. However, the development of abnormal disease symptoms was elevated in annual hosts and was significantly affected by the genetic distance between hosts-of-origin and inoculated annual species. Abnormal symptoms have been observed in cross-species inoculations and host shifts and are interpreted as a result of host specialization by Microbotryum (Antonovics et al. 2002; Lopez-Villavicencio et al. 2005; Sloan et al. 2008). Therefore, the high infection rates obtained here on annuals exposed to maladapted pathogens indicate a potentially more significant lack of physiological resistance than that inferred from observed infection rates alone. A long-term evolutionary consequence of this loss of physiological resistance may be to limit transitions from the annual to perennial habit in Silene, given the threat of acquiring anther smut in the absence of physiological defenses. Transitions between habits are frequent in the evolutionary history of the Silene genus, but unfortunately little is currently known regarding the directions of these evolutionary events (Hood et al. 2010).
The physiological susceptibility of annuals may also have a short-term impact on community-level disease dynamics. Pathogen spread and persistence is influenced by the interaction of multiple host populations that differ in their tolerance and competency as hosts (Fenton and Pedersen 2005; Hall et al. 2009; Kelly et al. 2009). As discussed above, highly susceptible annual plants can contract anther smut from sympatric diseased perennials and become transmissive within their single growing season. The movement of spores between sympatric annuals and perennials could augment transmission in perennial populations that are otherwise unsuitable for pathogen persistence (due to, e.g., high fragmentation, small population size, or high frequency of physiological resistance) (Carlsson-Granér and Thrall 2002; Gibson et al. 2010). Further studies of this disease in regions of high Silene species richness, with a mixture of annual and perennial habits, should be undertaken to determine the potential spillover and spillback dynamics of this disease.
In summary, we have complemented the many studies that examine the interactions of host ecology and infectious disease with experiments that assess the effects of host life history upon physiological resistance. Our results demonstrate that addressing the combination of physiological and ecological disease resistance in an appropriate model system provides insights into the impact of a pathogen introduction on novel host environments. Moreover, the phenomenon of ecological disease resistance explored in this study contributes to describing the complex phylogenetic and geographic patterns of distribution of an infectious disease in nature.
Conflict of Interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Identification of the Silene species used as annual and perennial hosts.
Table S2. Identification of Microbotryum species used in experimental inoculations.
Table S3. The relationship between the proportion infected by Microbotryum and flowering rate among families of the annual species Silene macrodonta.
References
- Agnew P, Bedhomme S, Haussy C, Michalakis Y. Age and size at maturity of the mosquito Culex pipiens infected by the microsporidian parasite Vavraia culicis. Proc. Biol. Sci. 1999;266:947–952. [Google Scholar]
- Agnew P, Koella J, Michalakis Y. Host life history responses to parasitism. Microbes Infect. 2000;2:891–896. doi: 10.1016/s1286-4579(00)00389-0. [DOI] [PubMed] [Google Scholar]
- Alexander HM. An experimental field study of anther-smut disease of Silene alba caused by Ustilago violacea: genotypic variation and disease incidence. Evolution. 1989;43:835–847. doi: 10.1111/j.1558-5646.1989.tb05181.x. [DOI] [PubMed] [Google Scholar]
- Alexander HM, Maltby A. Anther-smut infection of Silene alba caused by Ustilago violacea: factors determining fungal reproduction. Oecologia. 1990;84:249–253. doi: 10.1007/BF00318280. [DOI] [PubMed] [Google Scholar]
- Alexander HM, Antonovics J, Kelly AW. Genotypic variation in plant disease resistance–physiological resistance in relation to field disease transmission. J. Ecol. 1993;81:325–333. [Google Scholar]
- Alexander HM, Thrall PH, Antonovics A, Jarosz AM, Oudemans PV. Population dynamics and genetics of plant disease: a case study of anther-smut disease. Ecology. 1996;77:990–996. [Google Scholar]
- Altizer S, Nunn CL, Lindenfors P. Do threated hosts have fewer parasites? A comparative study in primates. J. Anim. Ecol. 2007;76:304–314. doi: 10.1111/j.1365-2656.2007.01214.x. [DOI] [PubMed] [Google Scholar]
- Antonovics J. Long-term study of a plant-pathogen metapopulation. In: Hanski I, Gaggiotti O, editors. Ecology, Genetics, and Evolution of Metapopulations. Burlington, MA: Academic Press; 2004. pp. 471–488. [Google Scholar]
- Antonovics J, Hood M, Partain J. The ecology and genetics of a host shift: Microbotryum as a model system. Am. Nat. 2002;160:S40–S53. doi: 10.1086/342143. [DOI] [PubMed] [Google Scholar]
- Biere A, Antonovics J. Sex-specific costs of resistance to the fungal pathogen Ustilago violaceaMicrobotryum violaceum) in Silene alba. Evolution. 1996;50:1098–1110. doi: 10.1111/j.1558-5646.1996.tb02350.x. [DOI] [PubMed] [Google Scholar]
- Biere A, Honders S. Host adaptation in the anther smut fungus Ustilago violaceaMicrobotryum violaceum): infection success, spore production and alteration of floral traits on two host species and their F1-hybrid. Oecologia. 1996;107:307–320. doi: 10.1007/BF00328447. [DOI] [PubMed] [Google Scholar]
- Bucheli E, Gautschi B, Shykoff JA. Differences in population structure of the anther smut fungus Microbotryum violaceum on two closely related host species, Silene latifolia and S. dioica. Mol. Ecol. 2001;10:285. doi: 10.1046/j.1365-294x.2001.01146.x. [DOI] [PubMed] [Google Scholar]
- Cafuir L, Antonovics J, Hood ME. Tissue culture and quantification of individual-level resistance to anther-smut disease in Silene vulgaris. Int. J. Plant Sci. 2007;168:415–419. [Google Scholar]
- Carlsson-Granér U, Thrall PH. The spatial distribution of plant populations, disease dynamics and evolution of resistance. Oikos. 2002;97:97–110. [Google Scholar]
- Charnov EL, Schaffer WM. Life-history consequences of natural selection: Cole's result revisited. Am. Nat. 1973;107:791–793. [Google Scholar]
- Cole LC. The population consequences of life history phenomena. Q. Rev. Biol. 1954;29:103–137. doi: 10.1086/400074. [DOI] [PubMed] [Google Scholar]
- Duffy MA, Ochs JH, Penczykowski RM, Civitello DJ, Klausmeier CA, Hall SR. Ecological context influences epidemic size and parasite-driven evolution. Science. 2012;335:1636–1638. doi: 10.1126/science.1215429. [DOI] [PubMed] [Google Scholar]
- Ezenwa V. Host social behavior and parasitic infection: a multifactorial approach. Behav. Ecol. 2004;15:446–454. [Google Scholar]
- Fenton A, Pedersen AB. Community epidemiology framework for classifying disease threats. Emerg. Infect. Dis. 2005;11:1815–1821. doi: 10.3201/eid1112.050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredensborg B, Poulin R. Parasitism shaping host life-history evolution: adaptive responses in a marine gastropod to infection by trematodes. J. Anim. Ecol. 2006;75:44–53. doi: 10.1111/j.1365-2656.2005.01021.x. [DOI] [PubMed] [Google Scholar]
- Gadgil M, Bossert W. Life historical consequences of natural selection. Am. Nat. 1970;104:1–24. [Google Scholar]
- Gaines MS, Vogt KJ, Hamrick JL, Caldwell J. Reproductive strategies and growth patterns in sunflowers (Helianthus. Am. Nat. 1974;108:889–894. [Google Scholar]
- Gibson AK, Mena-Ali JI, Hood ME. Loss of pathogens in threatened plant species. Oikos. 2010;119:1919–1928. [Google Scholar]
- Giraud T, Yockteng R, Lopez-Villavicencio M, Refregier G, Hood ME. Mating system of the anther smut fungus Microbotryum violaceum: selfing under heterothallism. Eukaryot. Cell. 2008;7:765–775. doi: 10.1128/EC.00440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold A, Giraud T, Hood M. Within-host competitive exclusion among species of the anther smut pathogen. BMC Ecol. 2009;9:11. doi: 10.1186/1472-6785-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Hayward AD, Watt KA, Pilkington JG, Pemberton JM, Nussey DH. Fitness correlates of heritable variation in antibody responsiveness in a wild mammal. Science. 2010;330:662–665. doi: 10.1126/science.1194878. [DOI] [PubMed] [Google Scholar]
- Hall SR, Becker C, Simonis J, Duffy M, Tessier A, Caceres C. Friendly competition: evidence for a dilution effect among competitors in a planktonic host-system. Ecology. 2009;90:791–801. doi: 10.1890/08-0838.1. [DOI] [PubMed] [Google Scholar]
- Hart R. Why are biennials so few? Am. Nat. 1977;111:792–799. [Google Scholar]
- Hawley DM, Lindstrom K, Wikelski M. Experimentally increased social competition compromises humoral immune responses in house finches. Horm. Behav. 2006;49:417–424. doi: 10.1016/j.yhbeh.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Hochberg M, Michalakis Y, de Meeus T. Parasitism as a constraint on the rate of life-history evolution. J. Evol. Biol. 1992;5:491–504. [Google Scholar]
- Hood ME. Dynamics of multiple infection and within-host competition by the anther-smut pathogen. Am. Nat. 2003;162:122–133. doi: 10.1086/375539. [DOI] [PubMed] [Google Scholar]
- Hood ME, Antonovics J. Intratetrad mating, heterozygosity, and the maintenance of deleterious alleles in Microbotryum violaceumUstilago violacea. Heredity. 2000;85:231–241. doi: 10.1046/j.1365-2540.2000.00748.x. [DOI] [PubMed] [Google Scholar]
- Hood ME, Mena-Alí JI, Gibson AK, Oxelman B, Giraud T, Yockteng R, et al. Distribution of the anther-smut pathogen Microbotryum on species of the Caryophyllaceae. New Phytol. 2010;187:217–229. doi: 10.1111/j.1469-8137.2010.03268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P, Rohr J, Hoverman J, Kellermanns E, Bowerman J, Lunde K. Living fast and dying of infection: host life history drives interspecific variation in infection and disease risk. Ecol. Lett. 2012;15:235–242. doi: 10.1111/j.1461-0248.2011.01730.x. [DOI] [PubMed] [Google Scholar]
- Jokela J, Lively C. Parasites, sex, and early reproduction in a mixed population of freshwater snails. Evolution. 1995;49:1268–1271. doi: 10.1111/j.1558-5646.1995.tb04453.x. [DOI] [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D, Paterson R, Townsend C, Poulin R, Tompkins D. Has the introduction of brown trout altered disease patterns in New Zealand fish? Freshw. Biol. 2009;54:1085–1818. [Google Scholar]
- Lafferty K. The marine snail, Cerithidea californica, matures and smaller sizes where parasitism is high. Oikos. 1993;68:3–11. [Google Scholar]
- Le Gac M, Hood ME, Fournier E, Giraud T. Phylogenetic evidence of host-specific cryptic species in the anther smut fungus. Evolution. 2007;61:15–26. doi: 10.1111/j.1558-5646.2007.00002.x. [DOI] [PubMed] [Google Scholar]
- Lee K, Wikelski M, Robinson W, Robinson T, Klasing K. Constitutive immune defences correlate with life-history variables in tropical birds. J. Anim. Ecol. 2008;77:356–363. doi: 10.1111/j.1365-2656.2007.01347.x. [DOI] [PubMed] [Google Scholar]
- Lindenfors P, Nunn CL, Jones KE, Cunningham AA, Sechrest W, Gittleman JL. Parasite species richness in carnivores: effects of host body mass, latitude, geographical range and population density. Glob. Ecol. Biogeogr. 2007;16:496–509. [Google Scholar]
- Lopez-Villavicencio M, Enjalbert J, Hood ME, Shykoff JA, Raquin C, Giraud T. The anther smut disease on Gypsophila repens: a case of parasite sub-optimal performance following a recent host shift? J. Evol. Biol. 2005;18:1293–1303. doi: 10.1111/j.1420-9101.2005.00924.x. [DOI] [PubMed] [Google Scholar]
- Lutz M, Platek M, Kemler M, Chlebicki A, Oberwinkler F. Anther smuts of Caryophyllaceae: molecular analyses reveal further new species. Mycol. Res. 2008;112:1280–1296. doi: 10.1016/j.mycres.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Miller MR, White A, Boots M. Host life span and the evolution of resistance characteristics. Evolution. 2007;61:2–14. doi: 10.1111/j.1558-5646.2007.00001.x. [DOI] [PubMed] [Google Scholar]
- Minchella D. Host life history variation in response to parasitism. Parasitology. 1985;90:205–216. [Google Scholar]
- Nunn CL, Altizer S, Jones KE, Sechrest W. Comparative tests of parasite species richness in primates. Am. Nat. 2003;162:597–614. doi: 10.1086/378721. [DOI] [PubMed] [Google Scholar]
- Ouborg NJ, Biere A, Mudde CL. Inbreeding effects on resistance and transmission-related traits in the Silene-Microbotryum pathosystem. Ecology. 2000;81:520–531. [Google Scholar]
- Oxelman B, Lidén M, Rabeler RK, Popp M. A revised generic classification of the tribe Sileneae (Caryophyllaceae) Nord. J. Bot. 2001;20:743–748. [Google Scholar]
- Pedersen AB, Grieves T. The interaction of parasites and resources cuase crashes in wild mouse population. J. Anim. Ecol. 2008;77:370–377. doi: 10.1111/j.1365-2656.2007.01321.x. [DOI] [PubMed] [Google Scholar]
- Pedersen AB, Jones KE, Nunn CL, Altizer S. Infectious diseases and extinction risk in wild mammals. Conserv. Biol. 2007;21:1269–1279. doi: 10.1111/j.1523-1739.2007.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright RK, Foley P, Field HE, Dobson AP, Foley JE, Eby P, et al. Urban habituation, ecological connectivity and epidemic dampening: the emergence of Hendra virus from flying foxes (Pteropus spp.) Proc. Biol. Sci. 2011;278:3703–3712. doi: 10.1098/rspb.2011.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp M, Oxelman B. Origin and evolution of North American polyploid Silene (Caryophyllaceae) Am. J. Bot. 2007;94:330–349. doi: 10.3732/ajb.94.3.330. [DOI] [PubMed] [Google Scholar]
- Rautenberg A, Sloan DB, Aldén V, Oxelman B. Phylogenetic relationships of Silene multinervia and Silene section Conoimorpha (Caryophyllaceae) Syst. Bot. 2012;37:226–237. [Google Scholar]
- Simms E. Costs of plant resistance to herbivory. Chicago, IL: University of Chicago; 1992. [Google Scholar]
- Sloan DB, Giraud T, Hood ME. Maximized virulence in a sterilizing pathogen: the anther-smut fungus and its co-evolved hosts. J. Evol. Biol. 2008;21:1544–1554. doi: 10.1111/j.1420-9101.2008.01604.x. [DOI] [PubMed] [Google Scholar]
- Swinfield T, Lewis O, Bagchi R, Freckleton R. Consequences of changing rainfall for fungal pathogen-induced mortality in tropical tree seedlings. Ecol. Evol. 2012;2:1408–1413. doi: 10.1002/ece3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Telfer S, Lambin X, Birtles R, Beldomenico P, Burthe S, Paterson S, et al. Species interactions in a parasite community drive infection risk in a wildlife population. Science. 2010;330:243–246. doi: 10.1126/science.1190333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrall PH, Biere A, Antonovics J. Plant life-history and disease susceptibility–the occurrence of Ustilago violacea on different species within the Caryophyllaceae. J. Ecol. 1993;81:489–498. [Google Scholar]
- Tinsley RC. The effects of host sex on transmission success. Parasitol. Today. 1989;5:190–195. doi: 10.1016/0169-4758(89)90144-0. [DOI] [PubMed] [Google Scholar]
- de Vienne D, Hood ME, Giraud T. Phylogenetic determinants of potential host shifts in fungal pathogens. J. Evol. Biol. 2009;22:2532–2541. doi: 10.1111/j.1420-9101.2009.01878.x. [DOI] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Orlando, Florida: Academic Press; 1990. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


