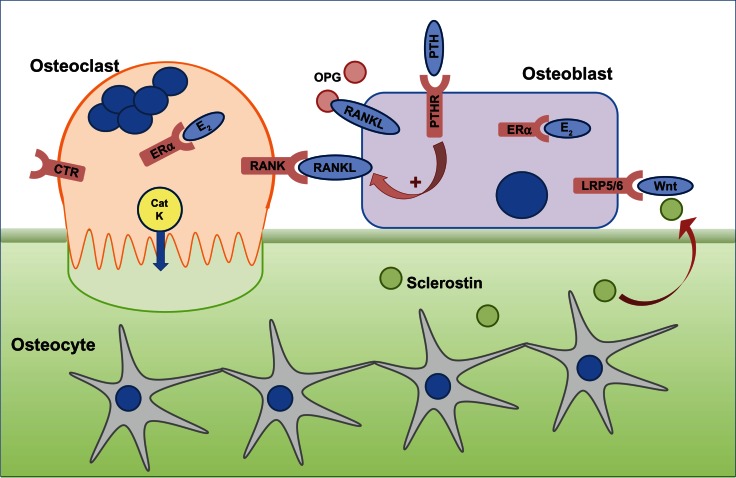
Figure 1.
The cells responsible for bone remodeling, highlighting key signaling pathways that are targets for therapies recommended for the prevention of osteoporotic fracture.
Notes: Osteocytes are embedded within mineralized bone and, in response to mechanical loading or microdamage, provide signals to osteoclasts to resorb. Osteoclast differentiation and function is dependent on the RANKL–RANK signaling pathway, which in vivo, is negatively regulated by OPG. Circulating PTH is a physiological regulator of plasma calcium and binds to PTHR on osteoblasts to indirectly stimulate osteoclast activity via upregulation of RANKL and downregulation of OPG expression. Calcitonin binds to the CTR expressed on mature osteoclasts to reversibly inhibit osteoclast function, although the exact physiological relevance for calcitonin is not fully understood. E2 has a positive effect on bone, through effects on osteoblasts and osteoclasts via ERα. CatK is secreted by resorbing osteoclasts across the convoluted ruffled border membrane and is required to degrade collagen. Osteoclast activity releases factors from the bone, which attract osteoblasts to the site of resorption. Osteoblast differentiation and function is controlled by the Wnt signaling pathway via the LRP5/6 and Frizzled co-receptors, which is regulated by endogenous inhibitors such as sclerostin, expressed by osteocytes and upregulated in response to unloading.
Abbreviations: CatK, cathepsin K; CTR, calcitonin receptor; E2, estrogen; ERα, estrogen receptor; LRP5/6, lipoprotein-related protein 5/6; OPG, osteoprotegerin; PTH, parathyroid hormone; PTHR, PTH receptor; RANK, receptor activator of nuclear factor-κB; RANKL, RANK ligand.