Abstract
Pulmonary artery aneurysm (PAA) is a rare entity. We report what we believe to be the first case of bronchiectasis resulting from a PAA, which in turn developed after a previous Senning procedure for transposition of the great vessels during infancy. The patient had bronchiectasis secondary to compression of the left main bronchus because of a PAA. Bronchiectasis is a condition indicating lung resection. Despite the patient receiving medical therapy to treat recurrent pneumonia, lobectomy was necessary to prevent this and other possible complications.
Keywords: Pulmonary artery aneurysm, Bronchiectasis, Congenital heart disease
INTRODUCTION
Pulmonary artery aneurysm (PAA) is a rare entity whose natural history is unknown. The main causes of PAA are pulmonary hypertension (PH) secondary to pulmonary embolus and to congenital heart disease with left–right shunts (the most common being patent ductus arteriosus). We report the first case, to our knowledge, of bronchiectasis resulting from a PAA.
CASE REPORT
A 24-year old woman, diagnosed at birth with D-transposition of the great vessels (D-TGA) with a ventricular septal defect (VSD), had severe stenosis at the origin of the right pulmonary branch and an aneurysmatic trunk. She underwent a Senning procedure with subpulmonary VSD closure and right pulmonary artery plasty when she was 1-year old. Within 10 years, she required further surgery due to a PAA that required a plication of the pulmonary trunk.
After several episodes of pneumonia, with abundant mucopurulent secretions, increasing dyspnoea from functional class II and presyncope, and failure of medical management (levofloxacin and bronchodilators), surgery was chosen. Auscultation revealed rales at the left base. Pulmonary function tests were: forced vital capacity 2511 ml (64%), forced expiratory volume in 1 s 1940 ml/s (61%), maximal mid-expiratory flow 39%, residual volume (RV) 178%, RV/total lung capacity 51%, diffusing capacity for carbon monoxide (DLCO) 60%, alveolar volume (VA) 81%, and DLCO/VA 76%.
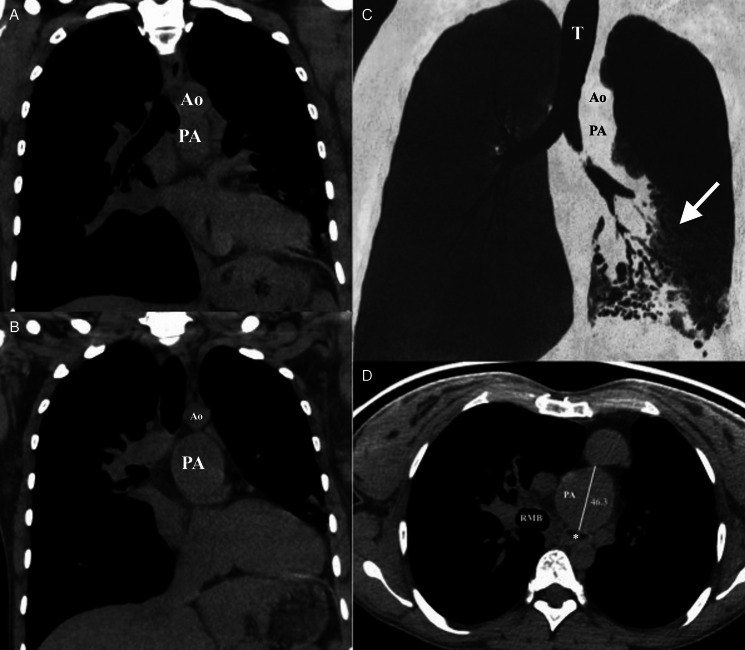
Echocardiography showed non-restrictive neo-atria, a small hyperdynamic left ventricle connected to the pulmonary artery, with systolic anterior motion of the mitral valve with no significant gradient, an enlarged pulmonary artery with a normally functioning valve, a systemic right ventricle connected to a moderately dilated aorta and hypertrophic systolic function at the low normal limit. A computed tomography (CT) scan showed bronchiectasis in the left lower lobe and a PAA with a diameter of 46.3 mm (Fig. 1). Pulsatile stenosis of the left main bronchus was evidenced on bronchoscopy (Fig. 2). Haemophilus influenzae and Staphylococcus aureus grew in the bronchial aspirate. The specific and single location of the bronchiectasis in the left lower lobe demonstrated the relationship between the pulmonary trunk aneurysm and the compression of the left main bronchus.
Figure 1:
(A and B) Thoracic CT scan (mediastinal window), coronal section: anatomical relationship between the left main bronchus and the aneurysm. (C) Minimum intensity projection (MinIP). Arrow shows specific and single location of bronchiectasis in the left lower lobe. (D) Thoracic CT scan (mediastinal window), axial section: aneurysm of the trunk PA compressing the left main bronchus (*). Diameter of the PA = 46.3 mm. PA: pulmonary artery; Ao: aorta artery; T: trachea; RMB: right main bronchus.
Figure 2:
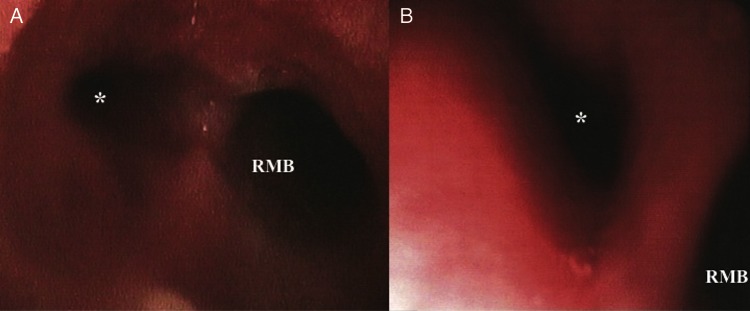
(A and B) Bronchoscopy: stenosis of the left main bronchus. RMB: right main bronchus. *Left main bronchus.
In view of the PAA and coexisting comorbidities, pulmonary trunk surgery was considered to entail a prohibitively high risk; hence, we limited the treatment to improving the quality of life and preventing complications. Surgery was performed under general anaesthesia and selective intubation. A left lateral thoracotomy was performed. The lobar pulmonary artery branches were normal. The pulmonary trunk was found to be dilated (diameter 5 cm). A conventional left lower lobectomy was performed with individual ligation and division of the lobar vessels and bronchi. The postoperative stay of 7 days was uneventful. The pathology study confirmed the bronchiectasis. Respiratory physiotherapy was instituted and, at 5 months of follow-up, the patient is asymptomatic with no further episodes of respiratory infection.
DISCUSSION
PAA is a rare entity. Deterling et al. [1] found 8 cases among 109 571 autopsies (0.0082%). The main aetiology of PAA is related to PH secondary pulmonary emboli or congenital heart disease with left–right shunts (the most common being patent ductus arteriosus). In our case, the aetiology was a D-transposition of the great vessels (D-TGA) corrected by the Senning technique. These procedures provide excellent results in the medium term, but can be associated with serious sequelae over the long term. The PAA is usually an incidental finding, but may be present with symptoms, such as dyspnoea, chest pain, haemoptysis, cyanosis, clubbing, cough and even hoarseness [2]. Our patient developed a PAA as a late complication of the Senning procedure. The PAA caused increasing dyspnoea and recurrent pneumonias due to stenosis of the left main bronchus.
Various techniques can be applied to cure a PAA: resection of the aneurysm followed by PA reconstruction with a tube (polytetrafluoroethylene, Dacron, a homograft or pericardium), aneurysmorrhaphy, or partial excision followed by patch reconstruction or reduction through a pulmonary arterioplasty [3]. In some cases, aneurysmectomy has been successfully replaced by allograft tissue [4]. In our patient, however, the previous operations resulted in an unfavourable anatomy, and pulmonary trunk surgery was considered to entail a prohibitively high risk. Thus, treatment was limited to the bronchiectasis.
Bronchiectasis is generally treated medically, reserving surgery for when medical treatment is not effective. Despite advances in medical treatment, some patients still need surgery. The current goals of surgical treatment for bronchiectasis are: to offer a cure, to improve the quality of life after failure of medical treatment and to solve and prevent complications such as empyema, severe haemoptysis and lung abscess. Whenever possible, a complete resection of localized disease should be performed, reserving palliative resections for diffuse bronchiectasis with severe localized disease [5]. The current philosophy of surgical treatment is to protect healthy tissue as much as possible and completely remove the diseased tissue. Some studies conclude that the optimal time to perform surgery is before the obstructive disease has developed, and that an early age of the patient may help to reduce postoperative morbidity and mortality, with surgery thus being more effective [4].
ACKNOWLEDGEMENT
Simona Espejo Pérez, Marta Blanco and Leandro José Burgos Vígara, Radiology Unit, Reina Sofía University Hospital, Córdoba.
Conflict of interest: none declared.
REFERENCES
- 1.Deterling RA, Jr, Clagett OT. Aneurysm of the pulmonary artery; review of the literature and report of a case. Am Heart J. 1947;34:471–99. doi: 10.1016/0002-8703(47)90527-9. doi:10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Bartter T, Irwin R, Nash G. Aneurysms of the pulmonary arteries. Chest. 1988;94:1065–75. doi: 10.1378/chest.94.5.1065. [DOI] [PubMed] [Google Scholar]
- 3.Vistarini N, Aubert S, Gandjbakhch I, Pavie A. Surgical treatment of a pulmonary artery aneurysm. Eur J Cardiothorac Surg. 2007;31:1139–41. doi: 10.1016/j.ejcts.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Araújo I, Escribano P, Lopez-Gude MJ, Jimenez C, Sanchez MA, Ruiz-Cano MJ, et al. BMC Cardiovasc Disord. 2011;11:64. doi: 10.1186/1471-2261-11-64. doi:10.1007/s11605-007-0264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agasthian T. Results of surgery for bronchiectasis and pulmonary abscesses. Thorac Surg Clin. 2012;22:333–44. doi: 10.1016/j.thorsurg.2012.04.008. doi:10.1016/j.ejso.2012.01.007. [DOI] [PubMed] [Google Scholar]