Abstract
Bacillus thuringiensis (Bt) produce inclusions that are composed of proteins known as crystal proteins or Cry toxins. Due to their high specificity and their safety to humans and the environment these Cry toxins are considered valuable alternatives to chemical pesticides in insect control programs. It is believed that Cry toxin-induced membrane pore formation is responsible for insect toxicity. The molecular mechanism of pore formation involves recognition and subsequent binding of the toxin to membrane receptors. This binding is accompanied by toxin oligomerization and transfer of domain I helices of the toxin to the lipid-water interface. This toxin insertion creates pores that lyse the cells. Several receptors from lepidopteran, coleopteran, and dipteran insects have been well characterized. Here we provide an overview of our understanding of the interactions between Cry toxin and multiple receptors in mosquitoes, in particular Aedes aegypti. We review the manner by which the receptors were identified and characterized, with a focus on three proteins – cadherin, alkaline phosphatase and aminopeptidase-N.
Keywords: Bacillus thuringiensis, Cry toxins, aminopeptidase, cadherin, alkaline phosphatase, receptor, Aedes aegypti
1. Cry toxin receptors
Bacillus thuringiensis (Bt) is a spore-forming pathogenic bacterium that distinguishes from other members of the Bacillus group because it produces crystalline inclusions known as Cry δ-endotoxins. The insecticidal properties of B. thuringiensis have been exploited worldwide for the control of insect vectors of human diseases and insect pests in agriculture. Due to the high selectivity and effectiveness of these toxins, their use surged dramatically following the introduction of cry genes into plants known as Bt crops (1, 2). Consequently it is important to elucidate theirs mode of action to ensure these toxins do not cause deleterious effects on human health and the environment (1, 3). Since Ae. aegypti is a principal mosquito vector of several diseases, including dengue and yellow fevers, this review deals primarily with the mode of action of Cry toxins in this mosquito species.
Upon ingestion by a susceptible mosquito larva, the alkaline midgut environment promotes solubilization of crystalline inclusions releasing the protoxins. Subsequent cleavage by gut proteases results in formation of active toxins. The activated toxin fragments then bind to specific protein receptors on midgut epithelial cells, leading to membrane insertion and pore formation. These pores allow ions and water to pass though the cells, resulting in swelling, lysis, and death of the insect (4-8). In the case of lepidopteran insects, sequential binding of Cry1A toxins has been reported. The binding mechanism may initially involve aminopeptidase-N (APN) and alkaline phosphatase (ALP) receptors followed by binding to a cadherin protein. Interaction with the cadherin protein triggers cleavage of helix α1, leading to the formation of oligomeric toxins (9). These oligomers then bind glycosylphosphatidylinositol (GPI)-anchored proteins in lipid rafts, including APN and ALP, resulting in the insertion of oligomeric toxins into the cell membrane (10, 11). An alternative model has been proposed that Cry1Ab toxins kill cells by a cascade of signal transduction events (12). In this model, Cry1Ab first binds the cadherin receptor. This interaction then stimulates a G protein and an adenylyl cyclase leading to an increase in cyclic AMP and protein kinase A levels, which consequentially leads to cellular alterations resulting in cell death. However, the preponderance of evidence supports the pore forming model of toxicity.
In any case, specific receptors are necessary for Cry toxin action. Four different protein receptors have been identified in lepidopteran insects – cadherin (13-15), APN (16, 17), ALP (18), and a 270 kDa glycoconjugate (19). In addition, glycolipids have been implicated (20). In mosquitoes, besides cadherin, APN, and ALP proteins, an α-amylase has been identified as a novel receptor in Anopheles albimanus, one of the malarial disease vectors (21).
Receptor expression levels have been shown to correlate with Cry toxin activity. For example, in Manduca sexta, the three identified protein receptors are expressed in the anterior, middle, and posterior regions of the midgut (22). These same regions also bind the Cry1A toxins (23). However, while the cadherin receptor protein was observed in all three regions, both the APN and ALP proteins were detected primarily in the posterior midgut. ALP was found at higher levels in the first and second larval instars, whereas APN was the main GPI-anchored Cry1Ab binding protein in the fourth and fifth instars (10). It appears that in early instars ALP plays a more important role in toxicity than APN proteins. Potentially these differences in expression patterns could explain the decreased susceptibility of late M. sexta larval instars to Cry1Ab (10). In addition, it has been reported that the presence of APN activity was not directly correlated with toxin binding (24). Moreover, no clear relationship could be found between APN activity and the toxicity of Cry proteins (25, 26), suggesting that the action of Cry toxins is dependent on their presence of protein receptors but not necessarily on their enzymatic activities (26).
However, interpretation of binding data may be obscured by irreversible associations of Cry toxins with BBMV, as well as its reversible associations (27-29). In fact, Cry toxin action is relatively complex likely involving more than one mechanism or one receptor. The idea of multiple receptor bindings may explain why toxin resistance has been linked to the mutations detected in either one of the protein receptors – cadherins (30, 31) or GPI-anchored protein such as APNs (32) and ALPs (18, 33). Taken together, these results show that cadherins, ALPs, and APNs are likely the potential receptors for Cry toxins. These receptor proteins will be discussed in more detail with respect to Cry toxin action in Ae. aegypti larval mosquitoes.
2. Potential receptors of Cry toxins in Aedes aegypti
2.1 Proteomic identification approaches
The larval mosquito midgut brush border has distinct structural elements, in which digestive enzymes, ion channel proteins and various extracellular matrices are located. Identification of the proteome of brush border membrane vesicles (BBMV) is a necessary step in defining potential Cry toxin receptors. Recently, a partial proteome of Ae. aegypti midgut BBMV was reported, in which a total of 119 proteins were identified using two complementary proteomic approaches (34). The most predominant proteins were arginine kinase, fatty acid binding protein, actin, aldehyde dehydrogenase and protein disulfide isomerase (34). Metallopeptidases with aminopeptidase activity and alkaline phosphatases, receptor molecules that serve as targets for Cry toxins (6, 10, 35-38), were also identified.
In an alternative approach, proteins separated by two-dimensional electrophoresis gel were probed with the Cry4Ba toxin. In this case the toxin bound three ALP isoforms and an aminopeptidase (39). Other Cry4Ba binding proteins included the lipid raft proteins, flotillin and prohibitin, the V-ATPase B subunit and actin. Generally, a cadherin–catenin complex forms a dynamic link with the actin filament network that is involved in the maintenance of cytoskeleton architecture in eukaryotic organisms (40). Potentially insertion of the Cry molecule into the membrane may expose regions of the toxin to the cytoplasm allowing contact with actin, which could lead to disruption of cytoskeletal links and loss of host cell shape and integrity (39, 41).
Identification of potential receptors through protein-protein interaction is another proteomics approach commonly used (17, 18, 42-44). Fernandez et al. reported that BBMV proteins purified from Cry11Aa ligand chromatography generated two proteins of 65 and 62 kDa, which bound the Cry11Aa toxin (36). In addition, the 62 kDa protein was a degraded product of the 65 kDa protein and both proteins were characterized as GPI-anchored ALP proteins (36). Recently, a pull-down assay using biotinylated Cry11Aa toxin as a bait purified three protein bands with molecular weights of 140, 95 and 45 kDa from Ae. aegypti larval midgut (45). With the exception of the 45 kDa actin, three of the proteins isolated were identified as APN, two with a mass of 95 kDa. However, the pull-down assay used in this study differed from that utilized by Fernandez et al, in which no APN proteins were observed (36). In this case biotinylated Cry11Aa toxin was incubated directly with BBMV, while Chen et al (45) incubated biotinylated Cry11Aa with a pool of proteins released from BBMV using phosphatidylinositol-specific phospholipase C (PI-PLC). Thus, differences in the methods used probably account for the separate identification of APN and ALP proteins (45). However, it is particularly noteworthy that the cadherin-like mosquito protein was not identified using the proteomic approaches utilized above (36, 39, 45-47). The high molecular mass of cadherins (200 kDa or more) or its stability could potentially affect its isolation using these approaches in mosquitoes. However, in lepidopteran insects these approaches led to identification of cadherin as a toxin receptor (13, 14).
2.2 Cadherins
Cadherins, are single-span transmembrane proteins located primarily within adherens junctions. They also belong to a family of calcium-dependent transmembrane glycoproteins. The presence of cadherins on the cell surface leads to cell sorting, cell adhesion, and morphogenesis. Specific interactions provided by extracellular regions can transfer information intracellularly by interacting with a complex network of cytoskeletal and signaling molecules (48). Cadherins have long been known as the Cry toxin receptors in a variety of insects in the orders Lepidoptera (butterflies and moths) (14, 49-51), Coleoptera (beetles and weevils) (52), and Diptera (mosquitoes) (47, 53). However, the cadherins that bind Cry toxins are distinct from other cadherins that are present within adherens junctions.
The toxin binding cadherins are localized in the insect midgut to regions in which toxin binding has been observed. In the larval midgut of Ae aegypti, cadherin distribution is observed on the apical side of the distal and proximal caeca and on posterior midgut epithelial cells but not in the apical membranes of anterior midgut (47). Further studies reveal that Cry4Ba and Cry11Aa toxins are also localized to these sites upon binding (47, 54). Thus, there appears to be a direct correlation between the binding pattern of Cry11Aa and Cry4Ba toxins and the localization of cadherin proteins leading us to believe that this cadherin serves as one of the main targets of Cry toxin binding within the mosquito gut.
The cadherin protein shown to interact with Cry11Aa toxin was a 250 kDa glycoprotein identified in Ae. aegypti BBMV (36). This protein, together with other two proteins of 100 and 65 kDa, was detected by ligand blot assay that bound Cry11Aa. The 65 kDa protein was later identified as an ALP protein (36). The cadherin protein plays a role in Cry11Aa toxin binding to Ae. aegypti midgut epithelia since an anti-AaeCad antibody could compete readily with Cry11Aa toxin binding to BBMV. In contrast, an antibody to the sodium-protein exchanger NHE3 that is also expressed in the midgut of Ae. aegypti mosquito, did not compete in the assay (47).
The Cry toxin binding cadherins have four distinct structural domains – a cytoplasmic domain, a transmembrane domain, a membrane proximal extracellular domain (MPED), and an ectodomain. The ectodomain in mosquitoes consists of 11 cadherin repeats (CR) instead of 12 repeats observed in moths and beetles. In lepidopteran insects the toxin-binding regions are primarily in the nearest cadherin repeats next to the MPED (55-57). Similarly, in Ae. aegypti, the toxin-binding domain was mapped to a C-terminal fragment that contains CR7 to CR11, with Cry11Aa having an affinity of ∼17 nM for this fragment (47). Within this C-terminal fragment, CR9-CR11 were found to bind Cry11Aa toxin through domain II loops α8 and 2. Furthermore, a Cry11Aa mutant in loop α8, E266A, was unable to bind a peptide fragment that contains CR9-11 (47). This binding is in agreement with previous work that showed a loop α8 peptide can compete with Cry11Aa binding to Ae. aegypti BBMV (58). With Cry11Ba, an anti-cadherin antibody also inhibited toxin binding to Ae. aegypti BBMV (59). A cadherin fragment consisting of CR7-CR11 was able to compete with Cry11Ba binding to BBMV. Hence, as with Cry11Aa, the Cry11Ba toxin binding region is likely localized to CR9-CR11 of the Ae aegypti cadherin receptor (60).
The importance of the CRs as a toxin-binding region was further studied by determining the correlation between binding and toxin susceptibility in different mosquito strains (53, 61). A CR11-MPED peptide from the cadherin of An. gambiae (AgCad1) larvae acted as a synergist of Cry4Ba's toxicity to the Anopheles mosquito (53). It is believed that this truncated cadherin peptide acts as a receptor, leading to cleavage of helix α1, thereby promoting formation of the oligomeric form of the toxin that binds the GPI-anchored receptors (62). Subsequently, it was demonstrated that the Anopheles CR9-11 and CR11-MPED fragments also enhanced Cry4Ba toxicity to Ae. aegypti larvae whereas a cadherin-based fragment isolated from a coleopteran insect, Diabrotica virgifera virgifera, did not affect Cry4Ba toxicity (61, 63). Both CR fragments were further tested for their binding affinity with Cry4Ba toxin. Using a one-site saturation model, it was shown that peptides CR9-11 and CR11-MPED bound Cry4B with high affinities of 13 and 23 nM, respectively (Table 1). Further the longer CR9-11 fragment was more potent than CR11-MPED in enhancing Cry4Ba activity against Ae. aegypti (61). Based on these results, these fragments can be used as synergists to increase Cry toxicity and potentially overcome insect resistance.
Table 1. Toxicity of purified Cry toxins to mosquito larvae and their binding affinity to midgut membrane proteins.
| Cry4Aa (93-95)a | Cry4Ba (61, 93, 94, 96) | Cry4Aa/Cry4Ba (93, 94) | Cry11Aa (45, 47, 96) | Cry11Ba (59, 60, 96) | |
|---|---|---|---|---|---|
| Ae. aegypti, LC50, ng/ml | 1360 | 300 | 280 | 122 | 7.9 |
| Binding to BBMV, Kd, nM | 99 | 41.6 | 3.6 | ||
| Binding to AeCadherin, Kd, nM | 16.7 | ||||
| Binding to AaeAPN1, Kd, nM | 8.5 | ||||
| An. gambiae, LC50, ng/ml | 1170 | 20 | 380 | 326 | |
| Binding to AgCadherin, CR9-11, Kd, nM | 13 | ||||
| Binding to AgCadherin, CR11-MPED, Kd, nM | 23 | ||||
| Binding to AgAPN1, Kd, nM | 23.9 | ||||
| An. quadrimaculatus | |||||
| Binding to APN, Kd, nM | 0.56 | ||||
| An. stephensi, LC50, ng/ml | 7400 | 550 | 300 | 372 | 2.2 |
| Cx. pipiens, LC50, ng/ml | 400 | >20000 | 63 | 10 | |
| Cx. quinquefasciatus, LC50, ng/ml | 980 | >80000 | 180 | 1140 | 3.3 |
| Binding to BBMV, Kd, nM | 20-30 |
References are in [brackets]
Recently an Ae. aegypti colony having a low level of resistance to Bt israelensis was identified from a field collection. Preliminary identification of resistance genes identified a N-cadherin as well as two other proteins including an APN as potential toxin targets (64). The N-cadherin is expressed in the larval midgut but is not known to bind any mosquitocidal toxins to date.
Mutations of mosquito cadherin genes will likely lead to lower larval sensitivity to single Cry toxins. However, unlike lepidopteran active strains, mosquitocidal active strains, such as Bt israelensis, produce multiple toxins with different modes of action. Consequently the development of high level mosquito resistance to these mosquitocidal strains has been lacking. In large part this lack of resistance development is due to the presence of Cyt1A, which acts to delay resistance development (65) by acting as a surrogate receptor for the mosquitocidal Cry toxins (66). In contrast, mosquito resistance to B. sphaericus has been rapid in the field (67-69).
2.3 ALPs
There is increasing evidence that ALPs are Cry toxin receptors in various insect species (10, 18, 36, 70-72). In the Ae. aegypti mosquito, preliminary reports suggested mosquitocidal Cry toxins bound proteins of 65 and 62 kDa (73, 74). Using a ligand blotting technique, the binding of biotinylated Cry toxins to these proteins was shown to be reversible, and both Cry4Ba and Cry11Aa toxins competed for binding to these two proteins (73). The 65 kDa protein lacked leucine aminopeptidase activity and the 62 kDa protein was a degradation product of the 65 kDa protein. Interestingly, Cry toxins inactive against Ae. aegypti larvae, such as the lepidopteran active Cry9Aa toxin, either fail to bind to the 65 and 62 kDa proteins or bind but did not compete for Cry11Aa toxin binding (74). Based on these results, the 65 and 62 kDa proteins are likely to be Cry4Ba and Cry11Aa toxin receptors in gut epithelial cells of Ae. aegypti larvae.
Further identification of ALP as Cry toxin receptors in Ae. aegypti was made possible by ligand blot analysis between Cry11Aa toxin and a pool of proteins released from BBMV by PI-PLC treatment (36). Three proteins of 200 kDa, 100 kDa, and 65 kDa were identified to bind the Cry11Aa toxin. The 65 kDa protein was purified by affinity chromatography with Cry11Aa toxin, and this protein was later characterized as a GPI-anchored ALP enzyme (70). The specific activity of this ALP was enriched up to 6-fold after PI-PLC treatment of BBMV and Cry11Aa affinity chromatography suggesting an abundance of ALP proteins in Ae. aegypti BBMV (36).
Immunofluorescence studies have shown that ALPs are located predominantly in gastric caeca and posterior midgut epithelial cells. The distribution pattern is similar to that of the cadherin protein and bound Cry11Aa toxin (47). Further studies have shown that phages displaying ALP-specific peptides decreased toxicity against Ae. aegypti larvae. Domain II loop α8 of Cry11Aa toxin was involved in the interaction with the ALP, since the binding of Cry11Aa and the displayed peptide phages was specifically attenuated by a peptide with a sequence corresponding to loop α8 (36). The putative ALP receptors have been subsequently cloned and characterized (70). Of three cloned ALPs, the ALP1 isoform (AAEL009077) was shown to bind Cry11Aa and the displayed peptide phage that specifically binds the midgut ALP-Cry11Aa receptor. Furthermore, two Cry11Aa regions (R59-G102 and N257-I296) that bind ALP1 were mapped by examining Cry11Aa binding to nine overlapping peptides of ALP1. By using a peptide spot array of the Cry11Aa domain III together with site-directed mutagenesis, it was shown that the ALP1 R59-G102 region binds Cry11Aa through domain II loop α8, while the ALP1 N257-I296 region interacts with Cry11Aa through domain III 561RVQSQNSGNN570 located in β18-β19. Conclusions drawn from these studies were that the Cry11Aa domains II and III are involved in binding two distinct binding sites in the ALP1 receptor (70).
Experiments carried out with Cry4Ba toxin and toxin-overlay assays were used to identify the toxin-binding BBMV protein complexes in Ae. aegypti (54). It was reported that domain II-III fragment reproducibly reacted with the same Ae. aegypti BBMV proteins as did the Cry4Ba toxin. One of these proteins was a 60 kDa protein, a size that approximates that of an ALP. However, the isolated domain III fragment did not bind these BBMV proteins, suggesting domain II of Cry4Ba toxin is essential for interaction with Ae. aegypti midgut proteins (54).
More recently, BBMV competitive assays revealed that the Cry11Ba binding to Ae. aegypti BBMV could be competed with Aedes ALPs. It was also demonstrated that AaeALP1 more readily competes off the binding of Cry11Ba toxin to BBMV than do AaeALP2 (AAEL000931) and AaeALP3 (AAEL003286), suggesting ALP1 could be more important in the interaction with Cry11Ba than the other ALP isoforms (60). Thus the ALP protein is an essential receptor molecule that mediates Cry11Aa toxicity and also is involved in the binding interaction of Cry4Ba and Cry11Ba with Ae. aegypti BBMV.
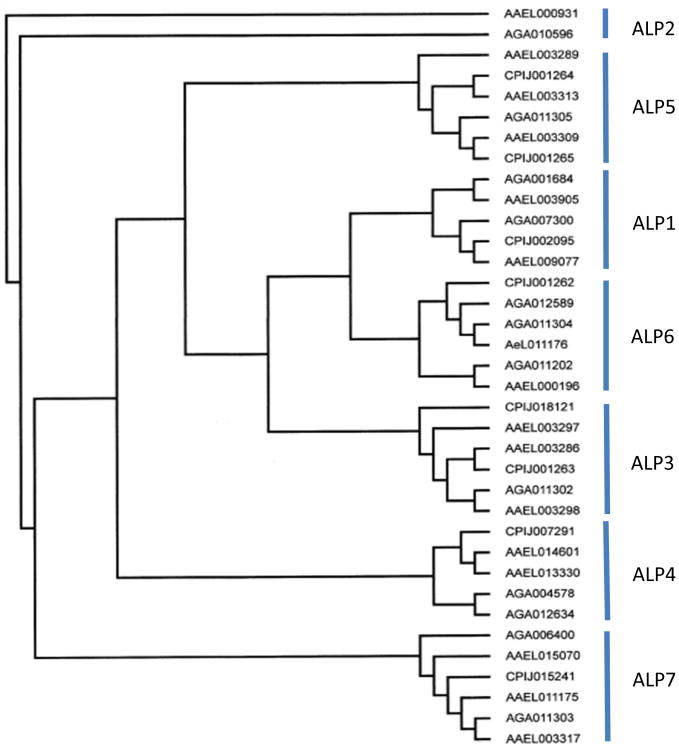
The diversity of ALPs present in mosquitoes is much larger than the three ALPs identified above. Depending on the level of diversity chosen, there are likely seven ALP families in mosquito species (Figure 1). Moreover, there appears to be significant gene diversity within these seven major ALP family classes. When compared with ALP sequences from other insects including Drosophila melanogaster, there is even more significant diversity among the ALP families (data not shown).
Figure 1.
Phylogenetic tree obtained from ClustalX alignment followed by bootstrap analysis using the maximum likelihood method of mosquito alkaline phosphatases. Protein sequences were obtained from Vectorbase for the three mosquito species – An gambiae (Agam Gene build 3.5), sequences have a prefix AGA; Aedes aegypti (AaegL Gene build 1.2), sequences have a prefix AAEL; and Culex pipiens (CpipJ Gene build 1.2), sequences have a prefix CPIJ. These prefixes are used as in noted in Vectorbase.
2.4 APNs
APNs are membrane proteins, whose function is to cleave amino acids at the N-terminus of polypeptides. It commonly serves, along with other enzymes, in the digestion of proteins derived from the insect's diet (75). These proteins have long been identified as Cry toxin receptors in various insect species (16, 76-84). Recently, a deletion mutation of the APN gene was associated with Cry1Ac resistance in Helicoverpa armigera (84) confirming that APN proteins may play an important role in the mechanism of Cry toxicity.
APNs require a signal peptide to direct nascent polypeptides to the outer surface of the cytoplasmic membrane, where they are attached by a GPI anchor (5, 85, 86). The APNs also undergo posttranslational modifications through N- and O-glycosylation, including that by N-acetylgalactosamine (GalNAc), which is considered to be important for interactions between Cry1A toxins and APNs (42, 87-89). However, some APNs are believed to bind toxins in a glycan-independent aspect as discussed below. Collectively these modifications give mature proteins of between 90 and 170 kDa in size, which affect the protein structure, stability, molecular recognition and signaling activities.
Less is known of APNs as Cry toxin receptors in mosquitoes. In An. quadrimaculatus and An. gambiae, APNs were identified as putative receptors for the Cry11Ba toxin (90, 91). Both of these APNs showed high affinity for the Cry11Ba toxin. For instance, an APN from An. quadrimaculatus binds Cry11Ba with a Kd of 0.56 nM, while a 106 kDa APN from An. gambiae binds the same toxin with an apparent affinity of 6.4 nM (Table 1). This is contrast to the binding affinity of Cry1A toxins to lepidopteran APN's that are in the range of 100 nM, suggesting that APN binding in mosquitoes may have a different role in the initial binding steps of mosquitocidal Cry toxins. It is believed that high affinity toxin binding occurs first to the more abundant GPI-anchored proteins and then to cadherin (Fig. 2). Nevertheless, no experimental evidence to date supports this possibility. A partial AgAPN2 fragment expressed in E. coli was able to bind Cry11Ba toxin in a dot blot experiment and a microtiter plate binding assay (91), suggesting this APN protein binds toxins in a glycan-independent manner. The 60 kDa APN (AgAPN2) from An. gambiae has only about a 46% homology to an APN from the sequenced genome (Agam P3.5 Gene Build, Vectorbase), while the An. quadrimaculatus APN has significant homology to a number of APNs from An. gambiae.
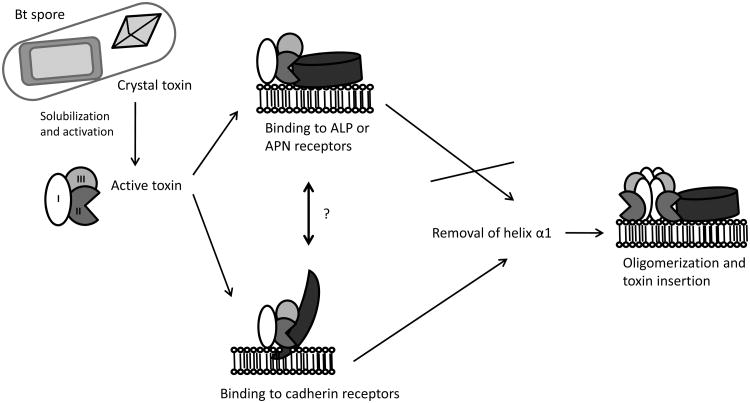
Figure 2.
Models of Cry toxin mechanism in mosquitoes. Upon ingestion by a mosquito larva, the alkaline midgut environment promotes solubilization of crystalline inclusions releasing the protoxins, which are cleaved by gut proteases resulting in formation of active toxins. Toxin binding occurs first with the cadherin protein triggering cleavage of helix α1, leading to the formation of oligomeric toxins, which then bind anchored APN or ALP proteins in lipid rafts, resulting in the insertion of oligomeric toxins into the cell membrane. Alternatively since mosquitocidal toxins have high affinity to APN and ALP receptors it is possible toxin binding initially involves APN and ALP receptors followed by binding to a cadherin protein.
The role of this receptor class was demonstrated only recently in Ae. aegypti(45). In this study, Ae. aegypti APNs, named AaeAPN1 (AAEL012778) and AaeAPN2 (AAEL008155), were isolated and identified as Cry11Aa-binding proteins in a biotinylated Cry11Aa toxin pull-down assay. As bait in the purifying process, Cry11Aa toxin bound four protein bands with molecular weights of 140, 95, 45, and 32 kDa (45). The 32 kDa protein is a fragment of Cry11Aa toxin. Three pulled down proteins were identified as APNs; the 140 kDa protein was AaeAPN1, while the 95 kDa consisted of two proteins identified as AaeAPN2 and AaeAPN3 (AAEL012774). AaeAPN1 was cloned and a partial fragment expressed in E. coli (45). This fragment was able to bind Cry11Aa suggesting the interaction of AaeAPN1-Cry11Aa is glycan-independent (45). Further studies revealed that the full-length AaeAPN2 and two of its fragments, AaeAPN2b and AaeAPN2e, bound Cry11Aa toxin and they also competed with Cry11Aa binding to Ae. aegypti BBMV. The data suggests amino acids 569-641 form part of the Cry11Aa toxin binding region in AaeAPN2 (Chen, J. and Gill, S.S. unpublished work). Similarly in AaeAPN1, one of the Cry11Aa-binding regions is localized to amino acids 525-778 (45). However, it should be noted that these regions in the two APNs are located toward the C-terminal part of the respective proteins. These binding regions differ from the observed Cry1Aa binding site in an Bombyx mori APN, which is localized to the N-terminal region (83, 92). Interestingly, Sf21 cells-expressing either AaeAPN1 or AaeAPN2 showed no increased sensitivity to Cry11Aa toxicity. It is noteworthy that the molecular weight of AaeAPN1 in Sf21 cells was lower than that of these proteins in BBMV. Therefore, it is possible that posttranslational modifications in Sf21 cells might differ from that observed in the epithelial cells of Ae. aegypti midgut and, also, improper glycosylations could affect toxin binding (45).
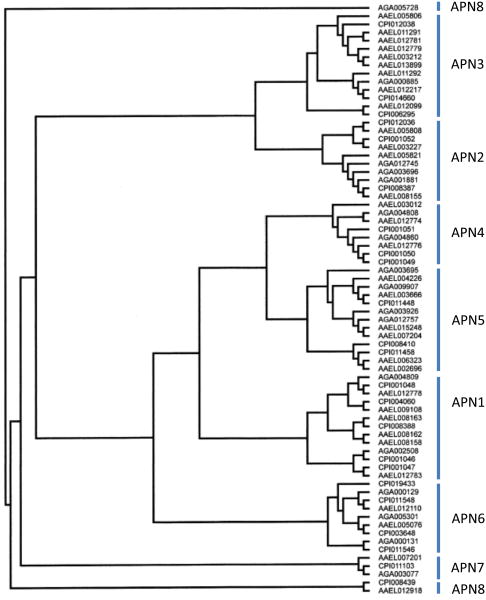
Depending on the level of divergence considered APNs can be divided into eight classes (91). It is evident, however, that mosquito APNs show significant differences from lepidopteran APNs. There is a great diversity in APNs among the three mosquito species whose genomes have been sequenced. Indeed phylogenetic analysis of An. gambiae, Ae. aegypti and Culex pipiens APNs shows that eight major families can be readily classified among these three mosquito species (Figure 3).
Figure 3.
Phylogenetic tree obtained from ClustalX alignment followed by bootstrap analysis using the maximum likelihood method of mosquito aminopeptidases. Protein sequences were obtained from Vectorbase for the three mosquito species –An gambiae (Agam Gene build 3.5), sequences have a prefix AGA; Aedes aegypti (AaegL Gene build 1.2), sequences have a prefix AAEL; and Culex pipiens (CpipJ Gene build 1.2), sequences have a prefix CPIJ.
3. Toxin receptor expression
Although high level receptor expression is observed in tissues and cell types that bind Cry toxins, the expression of cadherins, ALPs and APNs is not limited to these tissues. In fact all three receptor types are also expressed in the adult female midgut, and in the Malpighian tubules (data not shown). Clearly these proteins have functions which are critical for these tissues.
The tissue distribution patterns of the three receptor types have been examined in greater detail with immunofluorescence using specific antibodies. For example, Aedes cadherin has been localized to the apical side of the distal and proximal caeca and on the posterior midgut epithelial cells but its expression is not observed in the anterior midgut (47). This pattern of expression suggests some regionality in cadherin expression in the larval midgut. The cellular distribution in the adult midgut or in Malpighian tubules has not been determined. As noted earlier, AaeALP1 expression is similar to that of cadherin and hence it is expressed in gastric caeca and posterior midgut epithelial cells (47).
In the larval Aedes midgut AaeAPN1 showed a distinct expression pattern, with expression observed in the apical side of posterior midgut epithelial cells but not in the anterior midgut and gastric caeca cells (45). This expression pattern is similar to that observed in M. sexta gut, in which APN was preferentially expressed in the posterior gut epithelial cells. In contrast, AaeAPN2 was expressed not in posterior midgut cells but in the anterior midgut and gastric caeca cells (Chen, J., Aminova K.G., and Gill, S.S. unpublished work).
Microarray experiments performed using the gene set obtained from the recently sequenced Ae. aegypti genome was used to assess the effect of toxin exposure on the expression patterns of cadherins, ALPs and APNs. Low or high level toxin exposure had no effect on cadherin expression (data not shown). Similarly, the expression of AaeALP1 (AAEL009077), which bound Cry11Aa and also binds Cry11Ba, did not change following Cry11Aa exposure. In contrast, AaeALP2 (AAEL000931) and AaeALP4 (AAEL013330), which bind these toxins poorly showed significant decrease in expression levels following Cry11Aa exposure. A similar pattern was observed with APNs. It thus appears that toxin exposure had little effect on the receptor expression patterns.
Acknowledgments
We appreciate the technical assistance of Su-bum Lee. This research was funded in part through grants from the National Institutes of Health, 1R01 AI066014, DGAPA/UNAM IN218608 and IN210208-N, CONACyT U48631-Q and the University of California Agricultural Experiment Station.
Footnotes
Part of the symposium honoring Dr. John Casida. Sarjeet Gill was a Ph.D student with Dr. Casida 1969-1973
References
- 1.Shelton AM, Zhao JZ, Roush RT. Economic, ecological, food safety, and social consequences of the deployment of Bt transgenic plants. Annu Rev Entomol. 2002;47:845–81. doi: 10.1146/annurev.ento.47.091201.145309. [DOI] [PubMed] [Google Scholar]
- 2.Van Rie J. Bacillus thuringiensis and its use in transgenic insect control technologies. Int J Med Microbiol. 2000;290:463–9. doi: 10.1016/S1438-4221(00)80066-1. [DOI] [PubMed] [Google Scholar]
- 3.Tabashnik BE, Carriere Y, Dennehy TJ, Morin S, Sisterson MS, Roush RT, Shelton AM, Zhao JZ. Insect resistance to transgenic Bt crops: lessons from the laboratory and field. J Econ Entomol. 2003;96:1031–8. doi: 10.1603/0022-0493-96.4.1031. [DOI] [PubMed] [Google Scholar]
- 4.Knowles BH, Blatt MR, Tester M, Horsnell JM, Carroll J, Menestrina G, Ellar DJ. A cytolytic delta-endotoxin from Bacillus thuringiensis var. israelensis forms cation-selective channels in planar lipid bilayers. FEBS Lett. 1989;244:259–62. doi: 10.1016/0014-5793(89)80540-x. [DOI] [PubMed] [Google Scholar]
- 5.Pigott CR, Ellar DJ. Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol' Mol' Biol' Rev'. 2007;71:255–81. doi: 10.1128/MMBR.00034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bravo A, Gill SS, Soberon M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon. 2007;49:423–35. doi: 10.1016/j.toxicon.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bravo A, Miranda R, Gomez I, Soberon M. Pore formation activity of Cry1Ab toxin from Bacillus thuringiensis in an improved membrane vesicle preparation from Manduca sexta midgut cell microvilli. Biochim Biophys Acta. 2002;1562:63–9. doi: 10.1016/s0005-2736(02)00360-7. [DOI] [PubMed] [Google Scholar]
- 8.Jimenez-Juarez N, Munoz-Garay C, Gomez I, Gill SS, Soberon M, Bravo A. The pre-pore from Bacillus thuringiensis Cry1Ab toxin is necessary to induce insect death in Manduca sexta. Peptides. 2008;29:318–23. doi: 10.1016/j.peptides.2007.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez I, Sanchez J, Miranda R, Bravo A, Soberon M. Cadherin-like receptor binding facilitates proteolytic cleavage of helix alpha-1 in domain I and oligomer pre-pore formation of Bacillus thuringiensis Cry1Ab toxin. FEBS Lett. 2002;513:242–6. doi: 10.1016/s0014-5793(02)02321-9. [DOI] [PubMed] [Google Scholar]
- 10.Arenas I, Bravo A, Soberon M, Gomez I. Role of alkaline phosphatase from Manduca sexta in the mechanism of action of Bacillus thuringiensis Cry1Ab toxin. J Biol Chem. 2010;285:12497–503. doi: 10.1074/jbc.M109.085266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pacheco S, Gomez I, Arenas I, Saab-Rincon G, Rodriguez-Almazan C, Gill SS, Bravo A, Soberon M. Domain II loop 3 of Bacillus thuringiensis Cry1Ab toxin is involved in a “ping-pong” binding mechanism with Manduca sexta aminopetidase-N and cadherin receptors. J Biol Chem. 2009;284:32750–507. doi: 10.1074/jbc.M109.024968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Candas M, Griko NB, Taussig R, Bulla LA., Jr A mechanism of cell death involving an adenylyl cyclase/PKA signaling pathway is induced by the Cry1Ab toxin of Bacillus thuringiensis. Proc Natl Acad Sci USA. 2006;103:9897–902. doi: 10.1073/pnas.0604017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vadlamudi RK, Ji TH, Bulla LA., Jr A specific binding protein from Manduca sexta for the insecticidal toxin of Bacillus thuringiensis subsp. berliner. J Biol Chem. 1993;268:12334–40. [PubMed] [Google Scholar]
- 14.Nagamatsu Y, Toda S, Yamaguchi F, Ogo M, Kogure M, Nakamura M, Shibata Y, Katsumoto T. Identification of Bombyx mori midgut receptor for Bacillus thuringiensis insecticidal CryIA(a) toxin. Biosci Biotechnol Biochem. 1998;62:718–26. doi: 10.1271/bbb.62.718. [DOI] [PubMed] [Google Scholar]
- 15.Hara H, Atsumi S, Yaoi K, Nakanishi K, Higurashi S, Miura N, Tabunoki H, Sato R. A cadherin-like protein functions as a receptor for Bacillus thuringiensis Cry1Aa and Cry1Ac toxins on midgut epithelial cells of Bombyx mori larvae. FEBS Lett. 2003;538:29–34. doi: 10.1016/s0014-5793(03)00117-0. [DOI] [PubMed] [Google Scholar]
- 16.Gill SS, Cowles EA, Francis V. Identification, isolation, and cloning of a Bacillus thuringiensis CryIAc toxin-binding protein from the midgut of the lepidopteran insect Heliothis virescens. J Biol Chem. 1995;270:27277–82. doi: 10.1074/jbc.270.45.27277. [DOI] [PubMed] [Google Scholar]
- 17.Knight PJ, Knowles BH, Ellar DJ. Molecular cloning of an insect aminopeptidase N that serves as a receptor for Bacillus thuringiensis CryIA(c) toxin. J Biol Chem. 1995;270:17765–70. doi: 10.1074/jbc.270.30.17765. [DOI] [PubMed] [Google Scholar]
- 18.Jurat-Fuentes JL, Adang MJ. Characterization of a Cry1Ac-receptor alkaline phosphatase in susceptible and resistant Heliothis virescens larvae. Eur J Biochem. 2004;271:3127–35. doi: 10.1111/j.1432-1033.2004.04238.x. [DOI] [PubMed] [Google Scholar]
- 19.Valaitis AP, Jenkins JL, Lee MK, Dean DH, Garner KJ. Isolation and partial characterization of gypsy moth BTR-270, an anionic brush border membrane glycoconjugate that binds Bacillus thuringiensis Cry1A toxins with high affinity. Arch Insect Biochem Physiol. 2001;46:186–200. doi: 10.1002/arch.1028. [DOI] [PubMed] [Google Scholar]
- 20.Griffitts JS, Haslam SM, Yang T, Garczynski SF, Mulloy B, Morris H, Cremer PS, Dell A, Adang MJ, Aroian RV. Glycolipids as receptors for Bacillus thuringiensis crystal toxin. Science. 2005;307:922–25. doi: 10.1126/science.1104444. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Luna MT, Lanz-Mendoza H, Gill SS, Bravo A, Soberon M, Miranda-Rios J. An alpha-amylase is a novel receptor for Bacillus thuringiensis ssp. israelensis Cry4Ba and Cry11Aa toxins in the malaria vector mosquito Anopheles albimanus (Diptera: Culicidae) Environ Microbiol. 2010;12:746–57. doi: 10.1111/j.1462-2920.2009.02117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Brown MR, Hua G, Adang MJ. Comparison of the localization of Bacillus thuringiensis Cry1A delta-endotoxins and their binding proteins in larval midgut of tobacco hornworm, Manduca sexta. Cell Tissue Res. 2005;321:123–9. doi: 10.1007/s00441-005-1124-6. [DOI] [PubMed] [Google Scholar]
- 23.Bravo A, Hendrickx S, Jansen S, Peferoen M. Immunocytochemical Analysis of Specific Binding of Bacillus thuringiensis Insecticidal Crystal Proteins to Lepidopteran and Coleopteran Midgut Membranes. J Invertebr Pathol. 1992;60:247–254. [Google Scholar]
- 24.Jenkins JL, Lee MK, Sangadala S, Adang MJ, Dean DH. Binding of Bacillus thuringiensis Cry1Ac toxin to Manduca sexta aminopeptidase-N receptor is not directly related to toxicity. FEBS Lett. 1999;462:373–6. doi: 10.1016/s0014-5793(99)01559-8. [DOI] [PubMed] [Google Scholar]
- 25.Gilliland A, Chambers CE, Bone EJ, Ellar DJ. Role of Bacillus thuringiensis Cry1 delta Endotoxin Binding in Determining Potency during Lepidopteran Larval Development. Appl Environ Microbiol. 2002;68:1509–15. doi: 10.1128/AEM.68.4.1509-1515.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorence A, Darszon A, Bravo A. Aminopeptidase dependent pore formation of Bacillus thuringiensis Cry1Ac toxin on Trichoplusia ni membranes. FEBS Lett. 1997;414:303–7. doi: 10.1016/s0014-5793(97)01014-4. [DOI] [PubMed] [Google Scholar]
- 27.Liang Y, Patel SS, Dean DH. Irreversible binding kinetics of Bacillus thuringiensis CryIA delta-endotoxins to gypsy moth brush border membrane vesicles is directly correlated to toxicity. J Biol Chem. 1995;270:24719–24. doi: 10.1074/jbc.270.42.24719. [DOI] [PubMed] [Google Scholar]
- 28.Luo K, Banks D, Adang MJ. Toxicity, binding, and permeability analyses of four Bacillus thuringiensis cry1 delta-endotoxins using brush border membrane vesicles of spodoptera exigua and spodoptera frugiperda. Appl Environ Microbiol. 1999;65:457–64. doi: 10.1128/aem.65.2.457-464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu SJ, Dean DH. Functional significance of loops in the receptor binding domain of Bacillus thuringiensis CryIIIA delta-endotoxin. J Mol Biol. 1996;255:628–40. doi: 10.1006/jmbi.1996.0052. [DOI] [PubMed] [Google Scholar]
- 30.Xu X, Yu L, Wu Y. Disruption of a cadherin gene associated with resistance to Cry1Ac {delta}-endotoxin of Bacillus thuringiensis in Helicoverpa armigera. Appl Environ Microbiol. 2005;71:948–54. doi: 10.1128/AEM.71.2.948-954.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Chen H, Wu Y, Yang Y, Wu S. Mutated cadherin alleles from a field population of Helicoverpa armigera confer resistance to Bacillus thuringiensis toxin Cry1Ac. Appl Environ Microbiol. 2007;73:6939–44. doi: 10.1128/AEM.01703-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrero S, Gechev T, Bakker PL, Moar WJ, de Maagd RA. Bacillus thuringiensis Cry1Ca-resistant Spodoptera exigua lacks expression of one of four Aminopeptidase N genes. BMC Genomics. 2005;6:96. doi: 10.1186/1471-2164-6-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jurat-Fuentes JL, Adang MJ. Cry toxin mode of action in susceptible and resistant Heliothis virescens larvae. J Invertebr Pathol. 2006;92:166–71. doi: 10.1016/j.jip.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Popova-Butler A, Dean DH. Proteomic analysis of the mosquito Aedes aegypti midgut brush border membrane vesicles. J Insect Physiol. 2009;55:264–72. doi: 10.1016/j.jinsphys.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez I, Pardo-Lopez L, Munoz-Garay C, Fernandez LE, Perez C, Sanchez J, Soberon M, Bravo A. Role of receptor interaction in the mode of action of insecticidal Cry and Cyt toxins produced by Bacillus thuringiensis. Peptides. 2007;28:169–73. doi: 10.1016/j.peptides.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez LE, Aimanova KG, Gill SS, Bravo A, Soberon M. A GPI-anchored alkaline phosphatase is a functional midgut receptor of Cry11Aa toxin in Aedes aegypti larvae. Biochem J. 2006;394:77–84. doi: 10.1042/BJ20051517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ingle SS, Trivedi N, Prasad R, Kuruvilla J, Rao KK, Chhatpar HS. Aminopeptidase-N from the Helicoverpa armigera (Hubner) brush border membrane vesicles as a receptor of Bacillus thuringiensis crylac delta- endotoxin. Curr Microbiol. 2001;43:255–9. doi: 10.1007/s002840010297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parkin ET, Turner AJ, Hooper NM. Isolation and characterization of two distinct low-density, Triton- insoluble, complexes from porcine lung membranes. Biochem J. 1996;319:887–96. doi: 10.1042/bj3190887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bayyareddy K, Andacht TM, Abdullah MA, Adang MJ. Proteomic identification of Bacillus thuringiensis subsp. israelensis toxin Cry4Ba binding proteins in midgut membranes from Aedes (Stegomyia) aegypti Linnaeus (Diptera, Culicidae) larvae. Insect Biochem Mo l Biol. 2009;39:279–86. doi: 10.1016/j.ibmb.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Shimada Y, Usui T, Yanagawa S, Takeichi M, Uemura T. Asymmetric colocalization of Flamingo, a seven-pass transmembrane cadherin, and Dishevelled in planar cell polarization. Curr Biol. 2001;11:859–63. doi: 10.1016/s0960-9822(01)00233-0. [DOI] [PubMed] [Google Scholar]
- 41.Schlichting K, Wilsch-Brauninger M, Demontis F, Dahmann C. Cadherin Cad99C is required for normal microvilli morphology in Drosophila follicle cells. J Cell Sci. 2006;119:1184–95. doi: 10.1242/jcs.02831. [DOI] [PubMed] [Google Scholar]
- 42.Knight PJ, Crickmore N, Ellar DJ. The receptor for Bacillus thuringiensis CrylA(c) delta-endotoxin in the brush border membrane of the lepidopteran Manduca sexta is aminopeptidase N. Mol Microbiol. 1994;11:429–36. doi: 10.1111/j.1365-2958.1994.tb00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Charles CF, Darboux I, Pauron D, Nielsen-LeRoux C. Mosquitocidal Bacillus sphaericus: Toxins, Genetics, Mode of Action, Use, and Resistance Mechanisms. In: Gilbert LI, Kostas I, Gill SS, editors. Comprehensive Molecular Insect Science. Vol. 6. Elsevier; Oxford: 2005. pp. 207–31. [Google Scholar]
- 44.Darboux I, Nielsen-LeRoux C, Charles J, Pauron D. The receptor of Bacillus sphaericus binary toxin in Culex pipiens (Diptera: Culicidae) midgut: molecular cloning and expression. Insect Biochem Mo l Biol. 2001;31:981–90. doi: 10.1016/s0965-1748(01)00046-7. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Aimanova KG, Pan S, Gill SS. Identification and characterization of Aedes aegypti aminopeptidase N as a putative receptor of Bacillus thuringiensis Cry11A toxin. Insect Biochem Mo l Biol. 2009;39:688–96. doi: 10.1016/j.ibmb.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McNall RJ, Adang MJ. Identification of novel Bacillus thuringiensis Cry1Ac binding proteins in Manduca sexta midgut through proteomic analysis. Insect Biochem Mo l Biol. 2003;33:999–1010. doi: 10.1016/s0965-1748(03)00114-0. [DOI] [PubMed] [Google Scholar]
- 47.Chen J, Aimanova KG, Fernandez LE, Bravo A, Soberon M, Gill SS. Aedes aegypti cadherin serves as a putative receptor of the Cry11Aa toxin from Bacillus thuringiensis subsp. israelensis. Biochem J. 2009;424:191–200. doi: 10.1042/BJ20090730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Angst BD, Marcozzi C, Magee AI. The cadherin superfamily: diversity in form and function. J Cell Sci. 2001;114:629–41. doi: 10.1242/jcs.114.4.629. [DOI] [PubMed] [Google Scholar]
- 49.Gahan LJ, Gould F, Heckle DG. Identification of a gene associated with Bt resistance in Heliothis virescens. Science. 2001;293:857–860. doi: 10.1126/science.1060949. [DOI] [PubMed] [Google Scholar]
- 50.Morin S, Biggs RW, Sisterson MS, Shriver L, Ellers-Kirk C, Higginson D, Holley D, Gahan LJ, Heckel DG, Carriere Y, Dennehy TJ, Brown JK, Tabashnik BE. Three cadherin alleles associated with resistance to Bacillus thuringiensis in pink bollworm. Proc Natl Acad Sci USA. 2003;100:5004–9. doi: 10.1073/pnas.0831036100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vadlamudi RK, Weber E, Ji I, Ji TH, Bulla LA., Jr Cloning and expression of a receptor for an insecticidal toxin of Bacillus thuringiensis. J Biol Chem. 1995;270:5490–4. doi: 10.1074/jbc.270.10.5490. [DOI] [PubMed] [Google Scholar]
- 52.Fabrick J, Oppert C, Lorenzen MD, Morris K, Oppert B, Jurat-Fuentes JL. A novel tenebrio molitor cadherin is a functional receptor for Bacillus thuringiensis Cry3Aa toxin. J Biol Chem. 2009;284:18401–10. doi: 10.1074/jbc.M109.001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hua G, Zhang R, Abdullah MA, Adang MJ. Anopheles gambiae cadherin AgCad1 binds the Cry4Ba toxin of Bacillus thuringiensis israelensis and a fragment of AgCad1 synergizes toxicity. Biochemistry. 2008;47:5101–10. doi: 10.1021/bi7023578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moonsom S, Chaisri U, Kasinrerk W, Angsuthanasombat C. Binding characteristics to mosquito-larval midgut proteins of the cloned domain II-III fragment from the Bacillus thuringiensis Cry4Ba toxin. J Biochem Mol Biol. 2007;40:783–90. doi: 10.5483/bmbrep.2007.40.5.783. [DOI] [PubMed] [Google Scholar]
- 55.Chen J, Hua G, Jurat-Fuentes JL, Abdullah MA, Adang MJ. Synergism of Bacillus thuringiensis toxins by a fragment of a toxin-binding cadherin. Proc Natl Acad Sci USA. 2007;104:13901–6. doi: 10.1073/pnas.0706011104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griko NB, Rose-Young L, Zhang X, Carpenter L, Candas M, Ibrahim MA, Junker M, Bulla LA., Jr Univalent binding of the Cry1Ab toxin of Bacillus thuringiensis to a conserved structural motif in the cadherin receptor BT-R1. Biochemistry. 2007;46:10001–7. doi: 10.1021/bi700769s. [DOI] [PubMed] [Google Scholar]
- 57.Xie R, Zhuang M, Ross LS, Gomez I, Oltean DI, Bravo A, Soberon M, Gill SS. Single amino acid mutations in the cadherin receptor from Heliothis virescens affect its toxin binding ability to Cry1A toxins. J Biol Chem. 2005;280:8416–25. doi: 10.1074/jbc.M408403200. [DOI] [PubMed] [Google Scholar]
- 58.Fernandez LE, Perez C, Segovia L, Rodriguez MH, Gill SS, Bravo A, Soberon M. Cry11Aa toxin from Bacillus thuringiensis binds its receptor in Aedes aegypti mosquito larvae through loop alpha-8 of domain II. FEBS Lett. 2005;579:3508–14. doi: 10.1016/j.febslet.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 59.Likitvivatanavong S, Aimanova K, Gill SS. Loop residues of the receptor binding domain of Bacillus thuringiensis Cry11Ba toxin are important for mosquitocidal activity. FEBS Lett. 2009;583:2021–30. doi: 10.1016/j.febslet.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Likitvivatanavong S, Chen J, Bravo A, Soberon M, Gill SS. Analysis of the role of cadherin, alkaline phosphatase and aminopeptidase N as receptors of Cry11Ba toxin from Bacillus thuringiensis in Aedes aegypti. Appl Environ Microbiol. 2011;77 doi: 10.1128/AEM.01852-10. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park Y, Hua G, Abdullah MA, Rahman K, Adang MJ. Cadherin fragments from Anopheles gambiae synergize Bacillus thuringiensis Cry4Ba's toxicity against Aedes aegypti larvae. Appl Environ Microbiol. 2009;75:7280–2. doi: 10.1128/AEM.01870-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pacheco S, Gomez I, Gill SS, Bravo A, Soberon M. Enhancement of insecticidal activity of Bacillus thuringiensis Cry1A toxins by fragments of a toxin-binding cadherin correlates with oligomer formation. Peptides. 2009;30:583–8. doi: 10.1016/j.peptides.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park Y, Abdullah MA, Taylor MD, Rahman K, Adang MJ. Enhancement of Bacillus thuringiensis Cry3Aa and Cry3Bb toxicities to coleopteran larvae by a toxin-binding fragment of an insect cadherin. Appl Environ Microbiol. 2009;75:3086–92. doi: 10.1128/AEM.00268-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonin A, Paris M, Tetreau G, David JP, Despres L. Candidate genes revealed by a genome scan for mosquito resistance to a bacterial insecticide: sequence and gene expression variations. BMC Genomics. 2009;10:551. doi: 10.1186/1471-2164-10-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wirth MC, Georghiou GP, Federici BA. CytA enables CryIV endotoxins of Bacillus thuringiensis to overcome high levels of CryIV resistance in the mosquito, Culex quinquefasciatus. Proc Natl Acad Sci USA. 1997;94:10536–40. doi: 10.1073/pnas.94.20.10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perez C, Fernandez LE, Sun J, Folch JL, Gill SS, Soberon M, Bravo A. Bacillus thuringiensis subsp. israelensis Cyt1Aa synergizes Cry11Aa toxin by functioning as a membrane-bound receptor. Proc Natl Acad Sci USA. 2005;102:18303–8. doi: 10.1073/pnas.0505494102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nielsen-Leroux C, Charles JF, Thiery I, Georghiou GP. Resistance in a laboratory population of Culex quinquefasciatus (Diptera: Culicidae) to Bacillus sphaericus binary toxin is due to a change in the receptor on midgut brush-border membranes. Eur J Biochem. 1995;228:206–10. doi: 10.1111/j.1432-1033.1995.tb20251.x. [DOI] [PubMed] [Google Scholar]
- 68.Rao DR, Mani TR, Rajendran R, Joseph AS, Gajanana A, Reuben R. Development of a high level of resistance to Bacillus sphaericus in a field population of Culex quinquefasciatus from Kochi, India. J Am Mosq Control Assoc. 1995;11:1–5. [PubMed] [Google Scholar]
- 69.Silva-Filha MH, Nielsen-LeRoux C, Charles JF. Identification of the receptor for crystal toxin in the brush border membrane of the mosquito Culex pipiens (Diptera: Culicidae) Insect Biochem Mo l Biol. 1999;29:711–21. doi: 10.1016/s0965-1748(99)00047-8. [DOI] [PubMed] [Google Scholar]
- 70.Fernandez LE, Martinez-Anaya C, Lira E, Chen J, Evans A, Hernandez-Martinez S, Lanz-Mendoza H, Bravo A, Gill SS, Soberon M. Cloning and epitope mapping of Cry11Aa-binding sites in the Cry11Aa-receptor alkaline phosphatase from Aedes aegypti. Biochemistry. 2009;48:8899–907. doi: 10.1021/bi900979b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hua G, Zhang R, Bayyareddy K, Adang MJ. Anopheles gambiae alkaline phosphatase is a functional receptor of Bacillus thuringiensis jegathesan Cry11Ba toxin. Biochemistry. 2009;48:9785–93. doi: 10.1021/bi9014538. [DOI] [PubMed] [Google Scholar]
- 72.Sarkar A, Hess D, Mondal HA, Banerjee S, Sharma HC, Das S. Homodimeric alkaline phosphatase located at Helicoverpa armigera midgut, a putative receptor of Cry1Ac contains alpha-GalNAc in terminal glycan structure as interactive epitope. J Proteome Res. 2009;8(4):1838–48. doi: 10.1021/pr8006528. [DOI] [PubMed] [Google Scholar]
- 73.Krieger IV, Revina LP, Kostina LI, Buzdin AA, Zalunin IA, Chestukhina GG, Stepanov VM. Membrane proteins of Aedes aegypti larvae bind toxins Cry4B and Cry11A of Bacillus thuringiensis ssp. israelensis. Biochemistry (Mosc) 1999;64:1163–8. [PubMed] [Google Scholar]
- 74.Buzdin AA, Revina LP, Kostina LI, Zalunin IA, Chestukhina GG. Interaction of 65- and 62-kD proteins from the apical membranes of the Aedes aegypti larvae midgut epithelium with Cry4B and Cry11A endotoxins of Bacillus thuringiensis. Biochemistry (Mosc) 2002;67:540–6. doi: 10.1023/a:1015594127636. [DOI] [PubMed] [Google Scholar]
- 75.Wang P, Zhang X, Zhang J. Molecular characterization of four midgut aminopeptidase N isozymes from the cabbage looper, Trichoplusia ni. Insect Biochem Mo l Biol. 2005;35:611–20. doi: 10.1016/j.ibmb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 76.Atsumi S, Mizuno E, Hara H, Nakanishi K, Kitami M, Miura N, Tabunoki H, Watanabe A, Sato R. Location of the Bombyx mori aminopeptidase N type 1 binding site on Bacillus thuringiensis Cry1Aa toxin. Appl Environ Microbiol. 2005;71:3966–77. doi: 10.1128/AEM.71.7.3966-3977.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Banks DJ, Jurat-Fuentes JL, Dean DH, Adang MJ. Bacillus thuringiensis Cry1Ac and Cry1Fa delta-endotoxin binding to a novel 110 kDa aminopeptidase in Heliothis virescens is not N-acetylgalactosamine mediated. Insect Biochem Mo l Biol. 2001;31:909–18. doi: 10.1016/s0965-1748(01)00038-8. [DOI] [PubMed] [Google Scholar]
- 78.Crava CM, Bel Y, Lee SF, Manachini B, Heckel DG, Escriche B. Study of the aminopeptidase N gene family in the lepidopterans Ostrinia nubilalis (Hubner) and Bombyx mori (L.): Sequences, mapping and expression. Insect Biochem Mo l Biol. 2010;40:506–15. doi: 10.1016/j.ibmb.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 79.Hua G, Tsukamoto K, Rasilo ML, Ikezawa H. Molecular cloning of a GPI-anchored aminopeptidase N from Bombyx mori midgut: a putative receptor for Bacillus thuringiensis CryIA toxin. Gene. 1998;214:177–85. doi: 10.1016/s0378-1119(98)00199-1. [DOI] [PubMed] [Google Scholar]
- 80.Knight PJ, Carroll J, Ellar DJ. Analysis of glycan structures on the 120 kDa aminopeptidase N of Manduca sexta and their interactions with Bacillus thuringiensis Cry1Ac toxin. Insect Biochem Mo l Biol. 2004;34:101–12. doi: 10.1016/j.ibmb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 81.Lee MK, You TH, Young BA, Cotrill JA, Valaitis AP, Dean DH. Aminopeptidase N purified from gypsy moth brush border membrane vesicles is a specific receptor for Bacillus thuringiensis CryIAc toxin. Appl Environ Microbiol. 1996;62:2845–9. doi: 10.1128/aem.62.8.2845-2849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luo K, Sangadala S, Masson L, Mazza A, Brousseau R, Adang MJ. The Heliothis virescens 170 kDa aminopeptidase functions as “receptor A” by mediating specific Bacillus thuringiensis Cry1A delta-endotoxin binding and pore formation. Insect Biochem Mo l Biol. 1997;27:735–43. doi: 10.1016/s0965-1748(97)00052-0. [DOI] [PubMed] [Google Scholar]
- 83.Nakanishi K, Yaoi K, Nagino Y, Hara H, Kitami M, Atsumi S, Miura N, Sato R. Aminopeptidase N isoforms from the midgut of Bombyx mori and Plutella xylostella -- their classification and the factors that determine their binding specificity to Bacillus thuringiensis Cry1A toxin. FEBS Lett. 2002;519:215–20. doi: 10.1016/s0014-5793(02)02708-4. [DOI] [PubMed] [Google Scholar]
- 84.Zhang S, Cheng H, Gao Y, Wang G, Liang G, Wu K. Mutation of an aminopeptidase N gene is associated with Helicoverpa armigera resistance to Bacillus thuringiensis Cry1Ac toxin. Insect Biochem Mo l Biol. 2009;39:421–9. doi: 10.1016/j.ibmb.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 85.de Maagd RA, Bakker PL, Masson L, Adang MJ, Sangadala S, Stiekema W, Bosch D. Domain III of the Bacillus thuringiensis delta-endotoxin Cry1Ac is involved in binding to Manduca sexta brush border membranes and to its purified aminopeptidase N. Mol Microbiol. 1999;31:463–71. doi: 10.1046/j.1365-2958.1999.01188.x. [DOI] [PubMed] [Google Scholar]
- 86.Singh A, Sivaprasad C. Functional interpretation of APN receptor from M. sexta using a molecular model. Bioinformation. 2009;3:321–5. doi: 10.6026/97320630003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jenkins JL, Lee MK, Valaitis AP, Curtiss A, Dean DH. Bivalent sequential binding model of a Bacillus thuringiensis toxin to gypsy moth aminopeptidase N receptor. J Biol Chem. 2000;275:14423–31. doi: 10.1074/jbc.275.19.14423. [DOI] [PubMed] [Google Scholar]
- 88.Burton SL, Ellar DJ, Li J, Derbyshire DJ. N-acetylgalactosamine on the putative insect receptor aminopeptidase N is recognised by a site on the domain III lectin-like fold of a Bacillus thuringiensis insecticidal toxin. J Mol Biol. 1999;287:1011–22. doi: 10.1006/jmbi.1999.2649. [DOI] [PubMed] [Google Scholar]
- 89.Masson L, Lu YJ, Mazza A, Brousseau R, Adang MJ. The CryIA(c) receptor purified from Manduca sexta displays multiple specificities. J Biol Chem. 1995;270:20309–15. doi: 10.1074/jbc.270.35.20309. [DOI] [PubMed] [Google Scholar]
- 90.Abdullah MA, Valaitis AP, Dean DH. Identification of a Bacillus thuringiensis Cry11Ba toxin-binding aminopeptidase from the mosquito, Anopheles quadrimaculatus. BMC Biochem. 2006;7:16. doi: 10.1186/1471-2091-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang R, Hua G, Andacht TM, Adang MJ. A 106-kDa aminopeptidase is a putative receptor for Bacillus thuringiensis Cry11Ba toxin in the mosquito Anopheles gambiae. Biochemistry. 2008;47:11263–72. doi: 10.1021/bi801181g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yaoi K, Nakanishi K, Kadotani T, Imamura M, Koizumi N, Iwahana H, Sato R. Bacillus thuringiensis Cry1Aa toxin-binding region of Bombyx mori aminopeptidase N. FEBS Lett. 1999;463:221–4. doi: 10.1016/s0014-5793(99)01626-9. [DOI] [PubMed] [Google Scholar]
- 93.Angsuthanasombat C, Crickmore N, Ellar DJ. Comparison of Bacillus thuringiensis subsp. israelensis CryIVA and CryIVB cloned toxins reveals synergism in vivo. FEMS Microbiol Lett. 1992;73:63–8. doi: 10.1016/0378-1097(92)90584-b. [DOI] [PubMed] [Google Scholar]
- 94.de Barros Moreira Beltrao H, Silva-Filha MH. Interaction of Bacillus thuringiensis svar. israelensis Cry toxins with binding sites from Aedes aegypti (Diptera: Culicidae) larvae midgut. FEMS Microbiol Lett. 2007;266:163–9. doi: 10.1111/j.1574-6968.2006.00527.x. [DOI] [PubMed] [Google Scholar]
- 95.Delecluse A, Poncet S, Klier A, Rapoport G. Expression of cryIVA and cryIVB Genes, Independently or in Combination, in a Crystal-Negative Strain of Bacillus thuringiensis subsp. israelensis. Appl Environ Microbiol. 1993;59:3922–27. doi: 10.1128/aem.59.11.3922-3927.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Delecluse A, Rosso ML, Ragni A. Cloning and expression of a novel toxin gene from Bacillus thuringiensis subsp. jegathesan encoding a highly mosquitocidal protein. Appl Environ Microbiol. 1995;61:4230–35. doi: 10.1128/aem.61.12.4230-4235.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]