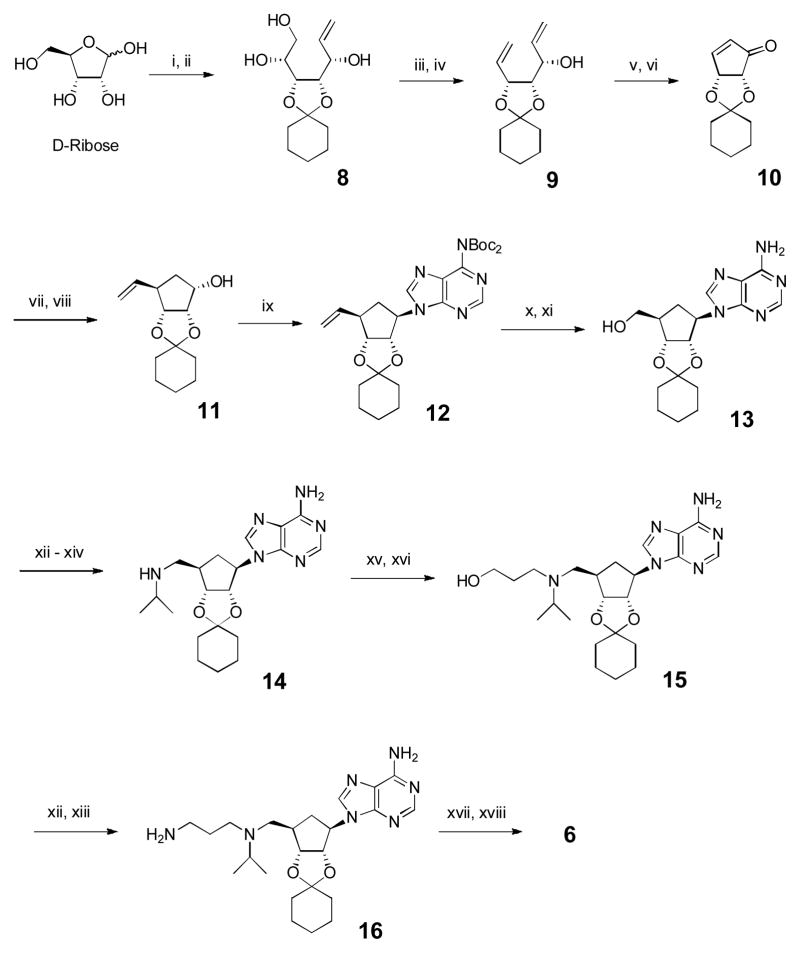
Scheme 1.
Synthesis for compound 6. Reagents and conditions: (i) cyclohexanone, cat. H2SO4; (ii) CH2=CHMgBr, THF, −78 °C, 70% for 2 steps; (iii) NaIO4, MeOH/H2O; (iv) Ph3PCH3Br, t-BuOK, THF, 87% for two steps; (v) 2nd generation Grubbs’ catalyst (5 mmol%), CH2Cl2; (vi) DMP, CH2Cl2, 86% for two steps; (vii) CH2=CHMgBr, TMSCl, hexamethylphosphor-amide, CuBr·Me2S, THF, −78 °C, 89% (viii) NaBH4, CeCl3·7H2O, MeOH, 0 °C, 96%; (ix) 6,6-di-Boc-adenine, Ph3P, DIAD, THF, 73%; (x) O3, CH2Cl2, −78 °C, then NaBH4/MeOH, 92%; (xi) trifluoroacetic acid, CH2Cl2, 96%; (xii) phthalimide, PPh3, DIAD, THF, 92%; (xiii) NH2NH2, EtOH, 80 °C, 99%; (xiv) acetone, NaCNBH3, MeOH, 95%; (xv) Methyl acrylate, MeOH, 65 °C, 99%; (xvi) LiAlH4, THF, −15 °C, 90%; (xvii) 4-tBuPhNCO, CH2Cl2, 95%; (xviii) HCl, MeOH, 92%.