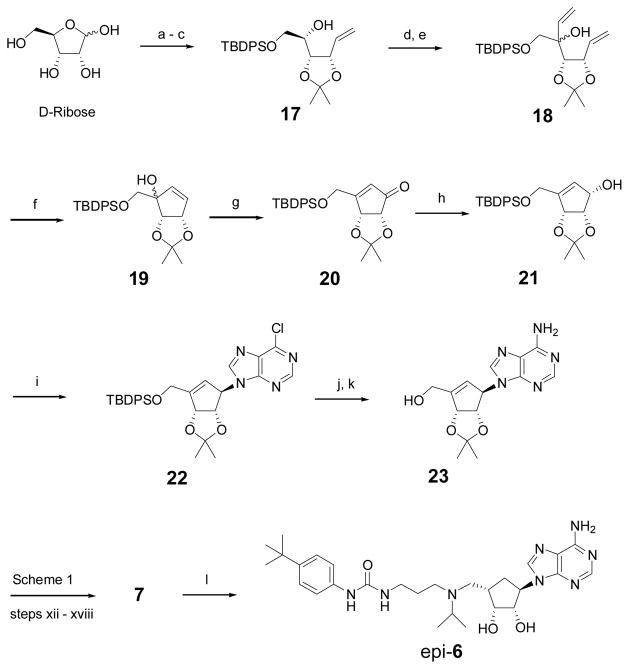
Scheme 2.
Synthesis for compounds 7 and epi-6. Reagents and conditions: (a) acetone, cat. H2SO4, 85%; (b) TBDPSCl, Et3N, 4-dimethylaminopyridine, DMF, 98%; (c) Ph3PMeBr, t-BuOK, THF, 92%; (d) SO3·Py, Et3N, CH2Cl2, 97%; (e) CH2=CHMgBr, THF, −78 °C, 93%; (f) 2nd generation Grubbs catalyst (5 mmol%), CH2Cl2, reflux, 95%; (g) PDC, 4 Å molecular sieve, DMF, 87%; (h) NaBH4, CeCl3·7H2O, MeOH, 0 oC, 98%; (i) 6-chloropurine, Ph3P, DIAD, THF, 95%; (j) 7 M NH3 in MeOH, 100 °C; (k) tetrabutylammonium fluoride, THF, 93% for two steps; (l) H2, 10% Pd/C, MeOH, 91%.