Table 6.
1,2-Halogen Migration/Cycloisomerization toward Halofurans 21
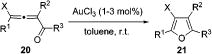
| Entry | Substrate | Time | Product | Yield (%)a | |
|---|---|---|---|---|---|
| 1 |
|
1 day |
|
21b | 75 |
| 2 |
|
1 day |
|
21c | 73 |
| 3 |
|
1 hr |
|
21d | 73 |
| 4 |
|
1 day |
|
21e | 61 |
| 5 |
|
0.5 hr |
|
21f | 88 |
| 6 |
|
3 days |
|
21g | 73 |
| 7 |
|
5 min |
|
21h | 97 |
| 8b |
|
1 hr |
|
21i | 71 |
| 9 |
|
3 days |
|
21j | 48 |
| 10b |
|
1 hr |
|
21k | 67c |
|
|
21l | ||||
Isolated yields, reactions were performed on 0.29–1 mmol scale with 1 M concentration of 20.
Mixture of allene and corresponding propargyl isomer was employed (see Supporting Information).
Mixture (2:1) of 21k and 21l by 1H NMR.
