Table 8.
Comparison of Lewis and Brønsted Acid Catalysts for Cycloisomerization of Allenyl Ketones
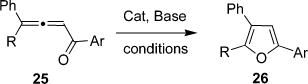
| entry | R | Ar | cat (mol %) | additive (mol %) | solvent | T, °C | time, h | NMR yield (%)a,b |
|---|---|---|---|---|---|---|---|---|
| 1 | Ph | p-C6H4-CN | TfOH (10) | – | toluene | 100 | 1.5 | 96 |
| 2 | Ph | p-C6H4-CN | TfOH (10) | TTBP (40) | toluene | 100 | 2.0 | > 99 |
| 3 | Ph | p-C6H4-CN | Sn(OTf)2 (5) | TTBP (20) | toluene | 100 | 2.0 | 91 |
| 4 | Ph | p-C6H4-CN | TfOH (20) | – | DCE | rt | 1.0 | 96 |
| 5 | Ph | p-C6H4-CN | TfOH (20) | TTBPc (40) | DCE | rt | 24 | 0 |
| 6 | Ph | p-C6H4-CN | TfOH (20) | TTBP (40) | DCE | 95 | 48 | 36 |
| 7 | Ph | p-C6H4-CN | TMSOTf (20) | – | DCE | rt | 1.0 | > 99 |
| 8 | Ph | p-C6H4-CN | TMSOTf (20) | TTBP (40) | DCE | rt | 4.0 | 0 |
| 9 | Ph | p-C6H4-CN | TMSOTf (20) | TTBP (40) | DCE | 95 | 24 | 61 |
| 10 | Ph | p-C6H4-CN | AgOTf (20) | – | DCE | 80 | 2.0 | > 99 |
| 11 | Ph | p-C6H4-CN | AgOTf (20) | TTBP (40) | DCE | 80 | 2.0 | 0 |
| 12 | Ph | p-C6H4-CN | AgOTf (20) | TTBP (40) | DCE | 95 | 48 | 19 |
| 13 | Ph | p-C6H4-CN | Sn(OTf)2 (5) | – | toluene | 100 | 1.5 | > 99 |
| 14 | Ph | p-C6H4-OMe | TfOH (10) | – | toluene | 100 | 1.0 | 88 |
| 15 | Ph | p-C6H4-OMe | In(OTf)3 (5) | – | toluene | 100 | 2.0 | 79 |
| 16 | Me | Ph | TfOH (10) | – | toluene | 100 | 1.0 | 52 |
| 17 | Ph | Ph | In(OTf)3 (5) | – | toluene | 100 | 12 | 77 |
Reactions were performed on 0.1 mmol scale.
Dibromomethane was used as the standard.
TTBP = 2,4,6-tris-tert-butylpyrimidine.
