Figure 3. Characterization of the anti-Arg1 antibody.
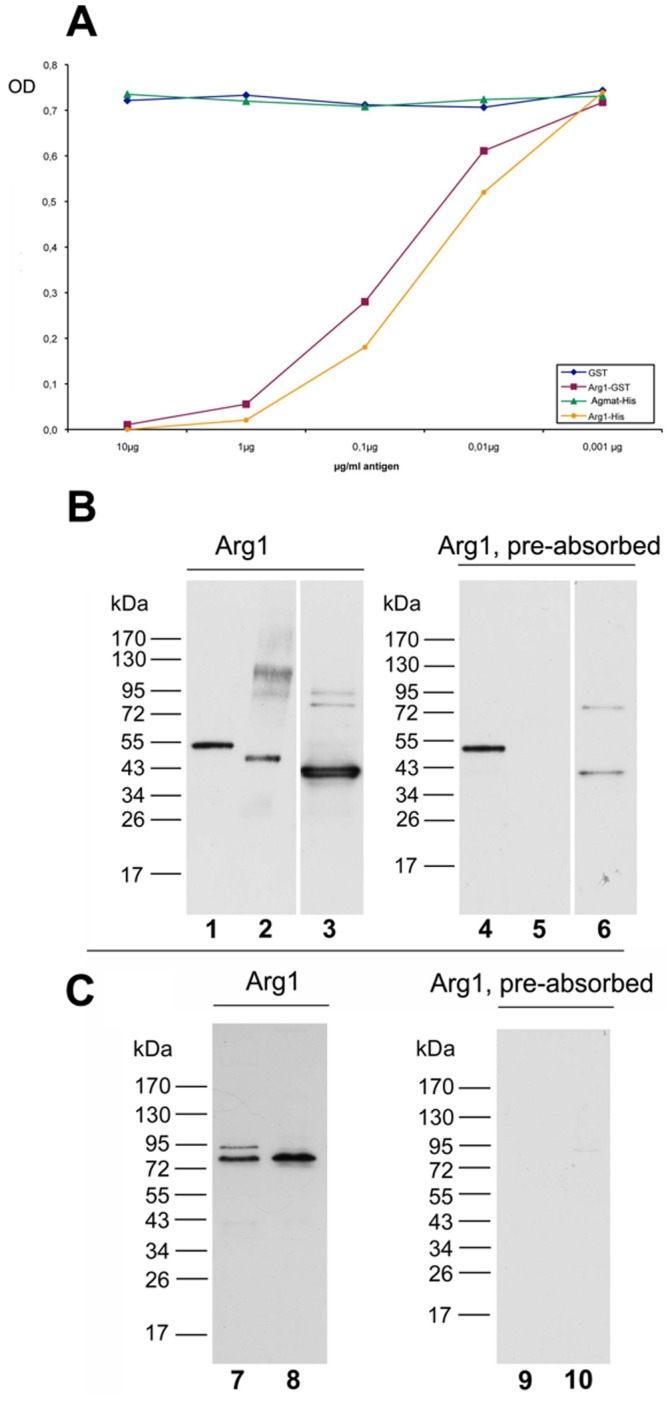
A ELISA assay displaying anti-Arg1 activity Competitive ELISA assay demonstrating the ability of different protein constructs to interfere with the anti-Arg1 immunosignal. Arg1-GST and Arg1-His at increasing concentrations competitively inhibited the specific interaction, demonstrating the antibodies reactivity against Arg1 fusion protein. In contrast, pre-adsorption using either Agm-His or -GST constructs did not show any effect on reactivity. B–C Characterization of the anti-Arg1 antibody by Western blotting. B: The anti-Arg1 antibody detected the bacterially expressed partial fusion proteins Arg1-GST (lane 1, 49,2 kDa) as well as Arg1-His (lane 2, 43,2 kDa). A strong 37kDa band was detectable in rat liver homogenate, a well known source rich of Arg1 (lane 3). C: In rat cortex homogenate (lane 7) and cytosol (lane 8) a band of about 80 kDa was detected. Pre-adsorption of the antibody with Arg1-His purified fusion protein (10 µg/ml lane 4–6, 40 µg/ml lane 9–10) lead to the disappearance of the observed bands, except for lane 4, which was loaded with Arg1-GST fusion protein. This reactivity could be attributed to residual anti-GST activity by testing the antibody against unconjugated GST protein (not shown). Loading: lane 1 and 4, Arg1-GST fusion protein (25 ng); lane 2 and 5, Arg1-His fusion protein (25 ng); lane 3 and 6, rat liver homogenate (25 µg); lane 7 and 9, rat cerebral cortex homogenate (40 µg); lane 8 and 10, rat cerebral cortex cytosol (40 µg).
