Figure 1. Dysfunctional adipose tissue in obesity drives β cell inflammation and T2D.
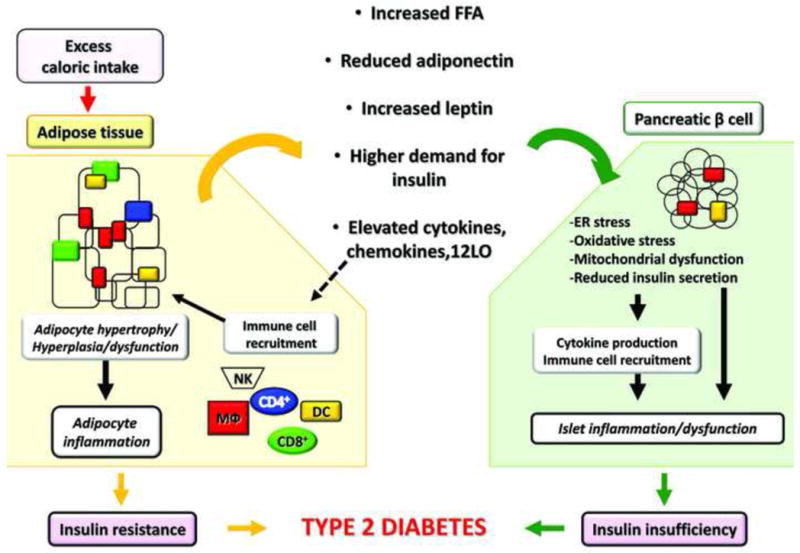
Excess caloric intake progressively induces weight gain and fat accretion leading to obesity. Adipose tissue (AT) is responsible for storage of ~90% of the FFA load following feeding, and therefore, substantial adipocyte remodeling involving primarily hypertrophy and some degree of hyperplasia occurs to accommodate the increasing demand for triglyceride storage. Hypertrophic adipocytes have significantly reduced response to insulin and become progressively more lipolytic liberating excessive FFA. Gene expression of inflammatory proteins and peptides increases resulting in elevated production of cytokines, chemokines, and other inflammatory mediators, such as 12LO. The latter, in turn, contributes to immune cell recruitment and activation within AT, including T cells (CD4+ and CD8+), MΦ, natural killer cells (NK), and dendritic cells (DC). Once in AT, immune cells are an additional source of pro-inflammatory mediators. The array of the adipokines secreted by the adipocytes also changes with increased production of leptin and reduced adiponectin. All of these circulating factors act in an endocrine manner and induce dysfunction in the pancreatic β cells. Elevated FFAs induce lipotoxicity, oxidative stress, mitochondrial dysfunction, and ER stress in the β cells; elevated leptin, coupled with leptin resistance in β cells, may contribute to reduced insulin secretion. Insulin secretion is further reduced by inflammatory mediators originating from either AT or locally produced by the islets and by the infiltrating immune cells. Adiponectin has beneficial effects via AdipoR1 and 2 receptors, which reduce oxidative stress and ER stress in the β cell. Reduced adiponectin expression in obesity minimizes its beneficial effects. Higher demand for insulin due to insulin resistance negatively affects β cells through ER stress and islet expansion. The recruitment of MΦ is also thought to amplify inflammation and islet dysfunction in obesity. It is yet to be determined whether other immune cells are involved in islet inflammation in T2D. The overall inflammatory responses in β cells in obesity contribute to reduced functional β cell mass leading to T2D, when combined with systemic insulin resistance.
